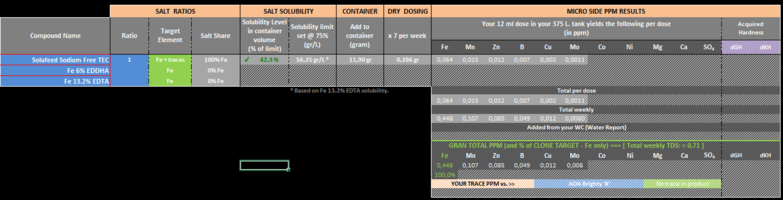
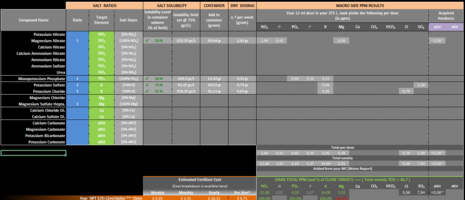
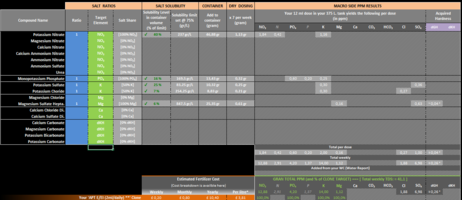
No, Calcium shouldn't be added to your fert bottle but instead dosed directly to your tank weekly if you are remineralizing your water of course. If you are using TAP water in your tank then it is possible it contains Ca already so it might not be necessary to add any Ca to the tank. Hopefully that's clearer.Hi Hanuman, I now have distilled water to use. That makes sense with the Calcium did I miss a warning in the excel document that said that Calcium shouldn't be dosed weekly?
Strange because the color of the water is quite clear and Fe tends to darken the water quite a bit. However I do notice that you are using very small amount of ferts so maybe that's the reason why the water is so clear.For the FE I am adding micro into the bottle I'm using the Aqua Plant Care CSM+B which I believe has Iron in it?
I'll answer that. YES. Do not incorporate Ca in your fertilizer mix. For Mg I did notice that you are adding quite a bit (~7.5ppm) so I interpret that as being your way of remineralizing the water in your tank. If that's the case then I would suggest also dry dosing Mg weekly like for Ca. This said, Mg will not react with the other compounds you have added in the your fertilizer.Would you suggest removing the Calcium from the mixture?
Perhaps you should clarify why you are adding that much Mg in the first place. Are you using RO in your tank or TAP water?