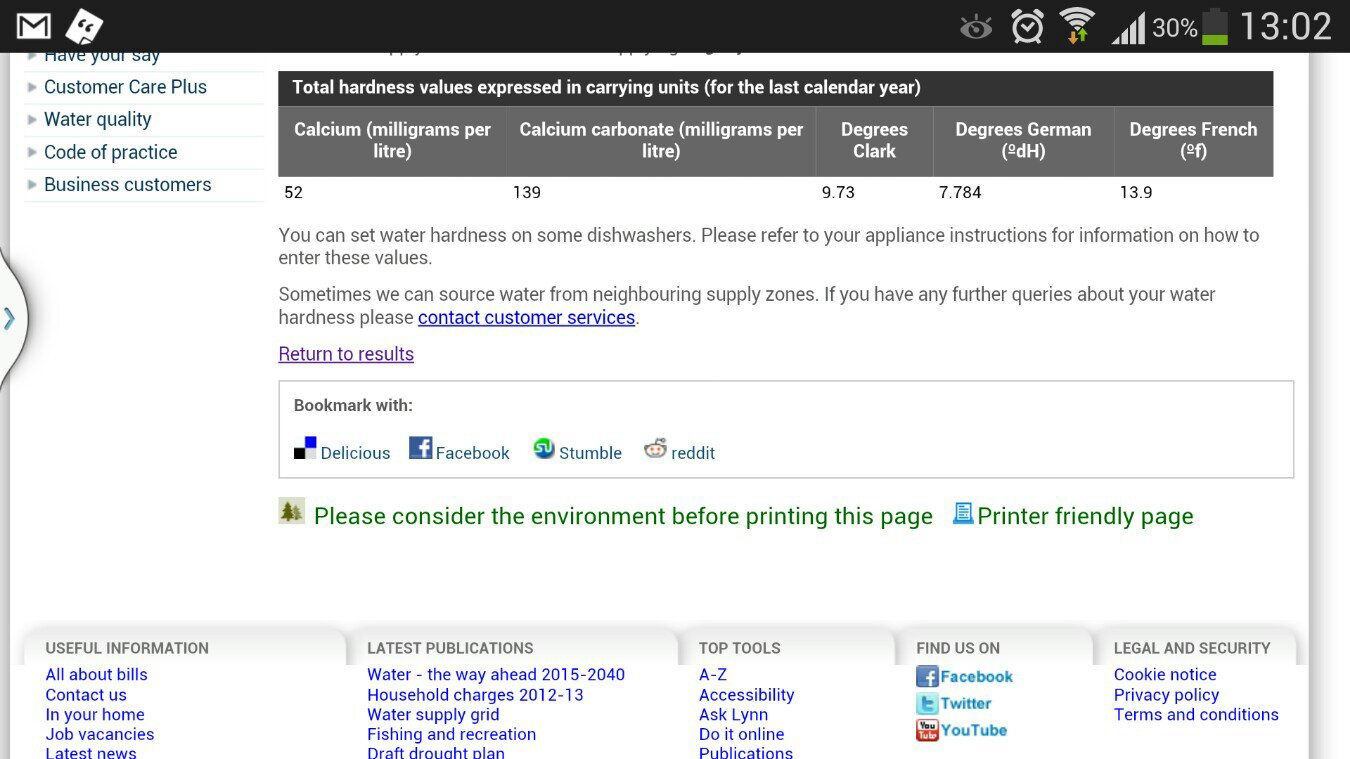
There is no direct correlation between KH and GH.
The measurement called KH is supposed to refer to the concentration level of Carbonate ions (CO3--) and the level of Bicarbonate ions (HCO3-) in the water. These ions are simply charged particles that have an imbalance of electric charge due to the migration and distribution of electrons (which are negatively charged) which are orbiting the positively charged nucleus.
Here is a schematic of the Carbonate ion. In this image the darker pink area of the structure indicates a strong negative charge due to 2 electrons that spend most of their time on the left side. The ion is said to be "polar" because the distribution of charge is not equal across the structure. Positive charges are therefore more likely to be attracted to that side of the structure. If the positive charge of a nearby particle is strong enough it will rip that Oxygen atom away from the CO3 structure and that original structure will become CO2.
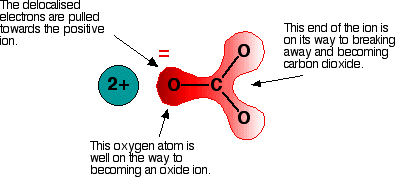
What can also happen is that a less strong positively charged nearby particle can attach itself to CO3. This changes the structure and the distribution of electrical charge as the electrons redistribute themselves. A single Hydrogen ion (H+) can attach itself to CO3 so that it becomes HCO3-. This new structure is called Bicarbonate, but it's new charge is -1 (as opposed to a -2 charge for CO3) because of the addition of the +1 charged Hydrogen. The new structure is drawn schematically as:
Still, both the -2 charged carbonate as well as the -1 charge Bicarbonate both strongly attract positively (+1) charged Hydrogen ions (H+).
Anytime there is an acid added to the water the acid structure disassembles to dump negatively charged particles in addition to H+ ions. The relative concentration of H+ determines the pH. A high positive charge in the water (due to high population of H+) will register a low pH. If there is an abundance of Carbonate or Bicarbonate in the water however, these negatively charged ions will tend to attract and sequester the H+ ions, thereby neutralizing the positive charge. This ability to sequester the H+, to reduce the impact of the positively charged ions from the acid is known as Alkalinity. Alkalinity in water suppresses the impact of H+ by capturing the H+ ions and attaching them to the two structures shown above.
This is why you take Bicarbonates such as Alka-Seltzer when you have an upset stomach due to high stomach acid content (when you eat a lot the stomach produces high levels of strong acids to break down the food). The "Alka" in the product name refers to Alkalinity.
So, water with high alkalinity means that there may be an abundance of CO3 and/or HCO3. I say "may be" because there are other positively charged particles that can sequester H+ in a similar manner to CO3 and HCO3. Your KH test kit has no idea which particles are actually performing this acid "buffering". The KH test kit therefore can only measure alkalinity NOT the Carbonate/Bicarbonate concentration because it cannot distinguish between the identity of the charged particles. It can only register their ability to neutralize an acid. Bicarbonate is a very important ion because the same thing it does for your stomach, it accomplishes in your blood (and in fishes blood) Because there is an interaction between bicarbonate, H+ and CO2, the circulatory system produces bicarbonate to neutralize acid in the bloodstream and to help move toxic CO2 away from the tissues to the lungs or gills where it can be ejected.
When you measure GH, what you are looking for is the waters content of the ions Magnesium (Mg+) and Calcium (Ca++). Again, the GH test kit cannot distinguish between these two, only their net effect in the water. Because these ions are positively charged, they can have some indirect effect on alkalinity, but these two parameters should not be linked. In some cases Calcium Carbonate (CaCO3) is added to the water, either intentionally or unintentionally. Because this compound disassociates to dump both Ca++ and CO3-- into the water, it will have an effect on GH as well as KH, but really there is no point in attempting to link the two, because these kits do not give you enough information to construct any link.
Cheers,