-
You are viewing the forum as a Guest, please login (you can use your Facebook, Twitter, Google or Microsoft account to login) or register using this link: Log in or Sign Up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
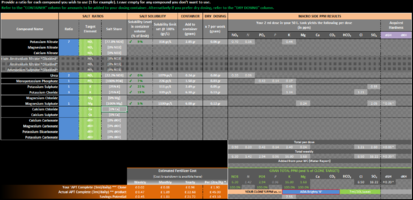
IFC Aquarium Fertilizer Calculator
- Thread starter Hanuman
- Start date
-
- Tags
- calculator clone diy fertilizer ppm
madhunm
Seedling
@Hanuman
The remineralizing wizards seem to be off by a factor of 100.
For example, I have set the water change to be 10L and set the calculator to clone Tetra pH/KH Plus (1dKH)
the results recommend me to doze 0.25g Potassium Carbonate into 10L of water to get 1dKH. Rotala Butterfly recommends that I add ~25g (24.62g actually) to 10L to get 1dKH. Screenshot attached

Cheers,
madhu.
The remineralizing wizards seem to be off by a factor of 100.
For example, I have set the water change to be 10L and set the calculator to clone Tetra pH/KH Plus (1dKH)
the results recommend me to doze 0.25g Potassium Carbonate into 10L of water to get 1dKH. Rotala Butterfly recommends that I add ~25g (24.62g actually) to 10L to get 1dKH. Screenshot attached

Cheers,
madhu.
Hi all,
Try the Rotala Butterfly calculator again, but put in "dry dosing" and "0.246g" of K2CO3. It is just more straightforward that way.
Your extra 100 (10^2) comes from the 200 mL "container size" and the 2 mL "dose size" added to the 10 litre tank .
cheers Darrel
No, it is back to the <"powers of 10">. I'm frequently <"lost in them">.The remineralizing wizards seem to be off by a factor of 100.
For example, I have set the water change to be 10L and set the calculator to clone Tetra pH/KH Plus (1dKH)
the results recommend me to doze 0.25g Potassium Carbonate into 10L of water to get 1dKH. Rotala Butterfly recommends that I add ~25g (24.62g actually) to 10L to get 1dKH.
Try the Rotala Butterfly calculator again, but put in "dry dosing" and "0.246g" of K2CO3. It is just more straightforward that way.
Your extra 100 (10^2) comes from the 200 mL "container size" and the 2 mL "dose size" added to the 10 litre tank .
cheers Darrel
Last edited:
Garuf
Member
So, I've taken a good run at this calculator lark but I can't get a sensible output for what I'm trying to make it do so perhaps I can be steered right.
I dose Tropica specialised and I'm noticing pin holing and yellowed out leaves which tells me po4 and iron and maybe k are a bit on the lean side, this would make sense as the water from the tap is right 'ard so also less available.
I want to bring my dosing up to say "lean Ei" and create a ml per litre, per day, number that I can then multiply to the tank volume.
Eg. my tanks are 18 and 38 so lets call it 20 and 40 with the filter volume. 40 X x = y Ml per day of Tropica specialised.
I dose Tropica specialised and I'm noticing pin holing and yellowed out leaves which tells me po4 and iron and maybe k are a bit on the lean side, this would make sense as the water from the tap is right 'ard so also less available.
I want to bring my dosing up to say "lean Ei" and create a ml per litre, per day, number that I can then multiply to the tank volume.
Eg. my tanks are 18 and 38 so lets call it 20 and 40 with the filter volume. 40 X x = y Ml per day of Tropica specialised.
water from the tap is right '
So is mine, and it recently came to my attention that the [Fe] and [Cu] in the tap water are within EU limits but vary over the year by 100 fold, plus excess in one element can result in symptoms of deficiency of another.
Checking your water companies water report may highlight any big fluctuations. Folk who use hard water have more issues and I think these fluctuations in trace elements may be contributing (if not the root cause) of the problems people have with hard water, the higher dGH and dKH may have nothing to do with the issues
you can use any of the preset regimes egI want to bring my dosing up to say "lean Ei" and create a ml per litre, per day, number that I can then multiply to the tank volume.
Eg. my tanks are 18 and 38 so lets call it 20 and 40 with the filter volume. 40 X x = y Ml per day of Tropica specialised.

or just set your own ppm targets

if you have entered you tank volume and dosing regime ( and water report details as well) the calculator will do the rest and report any issues on solubility of your fert solution
Garuf
Member
I think I must just be daft, I can't get it to put what I'm after, do I have to put in a water report to get it, because I don't have a water report I can easily lay my hands on.
The idea of the water report in the CoreSettings sheet is so that one can design a fertilizer in accordance to the tap water you are using in your tank. This basically allows you to customize your fertilizer to your own particular water report. Personally I think the Water Report function of the calculator should only be used if you know for a fact that a specific element is constant throughout the year, else don't use it. Simply create your fertilizer according to your preferred PPM targets in the TargetCalculator sheet.I think I must just be daft, I can't get it to put what I'm after, do I have to put in a water report to get it, because I don't have a water report I can easily lay my hands on.
Now, above you mentioned "Lean EI". What exactly is that? The calculator offers 4 types of DIY EI or other commercial fertilizers to choose from.

If those don't suit, then it's all good. You don't need to do a clone. Simply input the PPM you want by setting your own preferred targets as mentionned above by @Zeus.
If I didn't answer your question, please let me know.
Last edited:
I have detected a small inconsistency with "NilocG Thrive+ All-in-One" in the calculator. If you wish to clone that fert and want to follow the max guidelines of NilocG (Dose is 1 pump (2ml) per 10g, 1-3x per week Each) then in the calculator you need to "x dose by: 3" to have the proper ppm for a 3 times dosing per week. This minor issue was introduced in the latest version 1.07. All versions before that are correct. In reality this is not really an error but the calculator was designed in a way that we took the maximum advertised dosing of each commercial fertilizer in order to bring values closer to EI. This said, to allow users some flexibility the option "x-dose by:" is there in case one wants to reduce or increase the dosing easily.
I will correct this in the next version but wanted to give a heads up for anyone wanting to clone that fert.
I will correct this in the next version but wanted to give a heads up for anyone wanting to clone that fert.
Last edited:
I started to use this calculator and it’s really great, however I found it difficult in case you want to mix dry nutrients with some branded products.
basically I’m not able to put a data for GH booster. If you take a look on the original Tom Barr’s EI instruction you can add Seachem Equilibrium toghether with dry salts.
"1/4 teaspoon of KNO3 2-3x a week (every other day)
1/16th teaspoon of KH2PO4 2-3x a week (every other day)
Traces added on off days as the macro nutrients, so 3-5x a week, 5mls each time.
SeaChem Equilibrium or GH BOOSTER 1 teaspoon after water change"
basically I’m not able to put a data for GH booster. If you take a look on the original Tom Barr’s EI instruction you can add Seachem Equilibrium toghether with dry salts.
"1/4 teaspoon of KNO3 2-3x a week (every other day)
1/16th teaspoon of KH2PO4 2-3x a week (every other day)
Traces added on off days as the macro nutrients, so 3-5x a week, 5mls each time.
SeaChem Equilibrium or GH BOOSTER 1 teaspoon after water change"
Do you mean you can't target GH? If that's what you meant then you are right. The calculator at this point of time is not able to target GH directly, only Ca and Mg ppms separately. Maybe, I say maybe, this will come in the future as @Zeus. has been putting some hard work on it but no warranties on when and if it will be released with that feature. Any changes made to the calculator, even the smallest ones, usually requires from my side considerable amount of work due to quadruple checking/code re-writting/formatting/logical flow etc etc as I am very cautious about how the calculators look, its usability and user flow.I started to use this calculator and it’s really great, however I found it difficult in case you want to mix dry nutrients with some branded products.
basically I’m not able to put a data for GH booster. If you take a look on the original Tom Barr’s EI instruction you can add Seachem Equilibrium toghether with dry salts.
"1/4 teaspoon of KNO3 2-3x a week (every other day)
1/16th teaspoon of KH2PO4 2-3x a week (every other day)
Traces added on off days as the macro nutrients, so 3-5x a week, 5mls each time.
SeaChem Equilibrium or GH BOOSTER 1 teaspoon after water change"
Thank you for the awesome calculator. Great job to the member involved  it is really easy to use it.
it is really easy to use it.
I've been reading a lot on diy dry fert and trying to figure everything out until I stumbled this calculator. Make my life easier on calculation and finally brave enough and ordered like 8 ingredient to mess around which just arrived today but my weighting scale still haven't arrived yet. Going to start on DIY macro and fluval gro+micro first.
BTW is there a guideline on chloride ppm level? I ordered both KSO4 and KCL to make it more soluble.
Attached picture is the ratio I'm trying out. Do tell me if it have any problem.
I've been reading a lot on diy dry fert and trying to figure everything out until I stumbled this calculator. Make my life easier on calculation and finally brave enough and ordered like 8 ingredient to mess around which just arrived today but my weighting scale still haven't arrived yet. Going to start on DIY macro and fluval gro+micro first.
BTW is there a guideline on chloride ppm level? I ordered both KSO4 and KCL to make it more soluble.
Attached picture is the ratio I'm trying out. Do tell me if it have any problem.
Attachments
Thank you for the awesome calculator. Great job to the member involvedit is really easy to use it.
Thank you for you kind words, both myself and @Hanuman are happy you find it easy to use- Hani spent endless hours trying to make it easy to use. I think he did a great job on it
BTW is there a guideline on chloride ppm level? I ordered both KSO4 and KCL to make it more soluble.
The answer on that depends on who you are asking, both myself and 'Hani' dont class ourselves as fert experts, we are just the guys who did the program that does all the maths and enables everyone to compare ferts. However from the reading around which has been done in the R&D in making the program, I would aim for 50:50 Cl:SO4 ratio if I had all the salts to do it. I have recently got some KCl to enable me to this in the future. so its just a case of playing with the ratios of the salts till you happy with the weekly yields for Cl and SO4. Plus don't forget if your using tap water it also will contain Cl and SO4. So unless your using RO water it can be very hard to micro manage Cl and SO4 ( and any other elements as well).
So don't get to tied up in the details, if your plants look good your on target even if you just spoon the salts in the tank weekly
Glad to hear.Thank you for the awesome calculator. Great job to the member involvedit is really easy to use it.
Well chlorine I would say better to have 0ppm or you risk killing stuff. That's why we treat water coming from tap to neutralize all chlorine. Perhaps you were referring to chloride. I wouldn't get to tied up as @Zeus. said. I will also let the actual chemists answer with more precision.BTW is there a guideline on chloride ppm level?
Last edited:
Finally all arrived and I mixed 3 times ..2 times failed due to urea reacting with potassium chloride, producing some weird suspension. Read it up on some website saying urea is incompatible with it. So I cant use potassium chloride but, I've just realized my gh booster already had calcium chloride inside. My tap water has a reading of ph 6.5 kh gh <1 with TDS at 20ppm so I had to boost it, and which is why I was looking into including some tiny amount of chloride inside too in the first place. I'm not sure is it normal to have a TDS at 20ppm for tap water 😛 .Gonna start dosing and see the result, really loving the calculator
X3NiTH
Member
- Joined
- 13 Apr 2014
- Messages
- 1,669
mixed 3 times ..2 times failed due to urea reacting with potassium chloride, producing some weird suspension. Read it up on some website saying urea is incompatible with it
Im guessing you are using Tap water to mix the ferts. The TDS of your Tap Water is very low and if it’s being supplied municipally then it will be chock full of Orthophospate to moderate the pH upward to protect the distribution network.
The precipitation you are seeing will possibly be a form of Struvite and because the precipitation is slight it will remain as a suspension for a while. I would suggest you locate some RO/DI water for mixing this potion!

Impact of pH and Ionic Molar Ratios on Phosphorous Forms Precipitation and Recovery from Different Wastewater Sludges
🙂
Nope I used bottled RO water. and the formation of struvite is impossible as I used ascorbic acid and potassium sorbic in the beginning surely the ph would be around 3.
I just did a quick test on Potassium Chloride with the RO water on a small vial...it form the suspension. 😵😵😵 its food grade potassium chloride even. I'm lost lol
this is funny I tested the bottled RO TDS ...I get 100ppm gonna try other brand and probably distilled is better right
I just did a quick test on Potassium Chloride with the RO water on a small vial...it form the suspension. 😵😵😵 its food grade potassium chloride even. I'm lost lol
this is funny I tested the bottled RO TDS ...I get 100ppm gonna try other brand and probably distilled is better right
Last edited:
X3NiTH
Member
- Joined
- 13 Apr 2014
- Messages
- 1,669
100ppm that’s chock full of potential reactionary products, you want 0ppm tested RO/DI to show the true and fair representation of the mixture. As far as I’m aware Urea can modify ion crystal size formation but I’m assuming that would be for saturate solutions. If you can, please post the link for the article/s you saw on reactionary products between Urea and Potassium Chloride mixtures, if it’s something I’m not aware of then I want to know and be able to plan around it. Thanks!
Potassium and chloride in crops and soils: the role of potassium chloride fertilizer in crop nutrition
I found out why my food grade KCL make those suspension
and regarding urea+KCl ..a few source say limited compatibility, from the document above and
GUIDANCE FOR COMPATIBILITY OF FERTILIZER BLENDING MATERIALS
after unlocking a few paid research paper ... cant seems to find any reaction produced...
I found out why my food grade KCL make those suspension
Make sense that I found technical & technical grade is available here too and the solution of KCl I left overnight the it produces some thin layer of dust sediment on the bottom and becomes very clear. Not the usual thick precipitate. I wonder if I could just filter it out...Two undesirable properties of KCl are: (1) its tendency to cake and (2) dust formation which results from crystal breakage during handling and shipping. Therefore, agricultural grades are usually treated with additives (amines and oils) before shipping to improve their handling characteristics and to reduce caking and dusting (Eatock, 1985). When such additives are not wanted because of specific industrial demands, a drier and closely sized product (special grades) is shipped (UNIDO-IFDC, 1998).
and regarding urea+KCl ..a few source say limited compatibility, from the document above and
GUIDANCE FOR COMPATIBILITY OF FERTILIZER BLENDING MATERIALS
after unlocking a few paid research paper ... cant seems to find any reaction produced...