X3NiTH
Member
- Joined
- 13 Apr 2014
- Messages
- 1,669
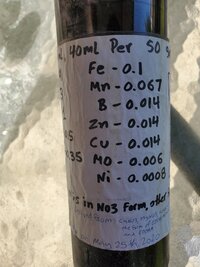
@Vsevolod Stakhov is probably experiencing whatever I experience from time to time while using Asrobic acid. I think i described this issues in one of the thread. my only guess is that the more Asrobic acid we add, it break down the Chelate and possibly precipitate the Iron and other elements, this occurred even while using a pure Distilled water to make the solution.
Fundamentally what is happening is that increased acid addition is changing the solution charge equilibrium toward an increase in protonation, the solution therefore is out of charge balance and as such there will be reactions between the elements within the solution to bring it back into balance even when all compounds are below their solubility threshold. More complex acids like Ascorbic and Acetic introduce carbon into the equation which will form part of reaction products to balance the charge, this would increase the chances of carbonate compound formations which can be fairly insoluble, a simple acid like HCl reduces the complexity of the charge balance equation and if compounds are formed they will be more likely chlorides which are very soluble so the chances for precipitative effects are much more reduced.
You can calculate the charge balance for a pH moderated solution of chosen compound molar concentration beforehand to reduce the likelihood of precipative effects but it becomes a very Complex Equilibrium Equation as you add more compounds in differing molar concentration.
The document below is an exceptional read and has all the equations within to calculate the above and so much more!
W. M. White Geochemistry Chapter 6: Aquatic Chemistry
🙂