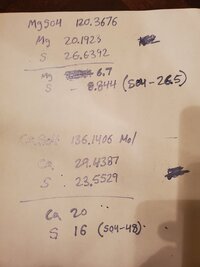
Either am loosing my mind today or something still isn't adding up. I get the following:The hydrate forms. CaSO4·2H2O and MgSO4‧7H2O which is what I use.
View attachment 183197
-
You are viewing the forum as a Guest, please login (you can use your Facebook, Twitter, Google or Microsoft account to login) or register using this link: Log in or Sign Up
Remineralizing question
- Thread starter Hanuman
- Start date
I think I figured it out. The calculator was adding up the extra oxygen from the hydrate (7H2O - 11 atoms of O instead of only 4) to SO4 when a hydrate was selected which should not happen. No matter the hydrate, SO4 and Ca should remain constant. Only the required mass changes. This will require an immediate update and release as I view this as an important fix. I will discuss with @Zeus. first so that we are on the same page.
@Happi Thanks for pointing this out.
@Happi Thanks for pointing this out.
Last edited:
Make sense, I thought I was going crazy 🤪 today.I think I figured it out. The calculator was adding up the extra oxygen from the hydrate (7H2O - 11 atoms of O instead of only 4) to SO4 when a hydrate was selected which should not happen. No matter the hydrate, SO4 and Ca should remain constant. Only the required mass changes. This will require and immediate update and release as I view this as an important fix. I will discuss with @zeus first so that we are on the same page.
@Happi Thanks for pointing this out.
Hi all,
All of chloride containing salts, that we might be interested in, <"are soluble">, which would be an advantage. The disadvantage is that chloride (Cl-) is monovalent, meaning that you need two of them when you add a divalent cation, so calcium chloride is CaCl2.nH2O etc.
Sulphates are a mixed bag in terms of solubility, but again it is only calcium sulphate (CaSO4.nH2O), that is both of interest to us and although not actually insoluble is "sparingly soluble".
For divalent cations sulphates are probably best, so magnesium sulphate (MgSO4.nH2O) rather than magnesium chloride (MgCl2).
The advantage of nitrate (potassium nitrate (KNO3 etc.)) and phosphate compounds (dipotassium phosphate (K2PO4) etc) would be both cation and anion are plant nutrients.
cheers Darrel
Plants don't have much requirement for either sulphur (S) or chlorine (Cl), so ideally you would want to add as little a you can compared to the metal ion that you do want e.g. potassium (K), magnesium (Mg) etc.If I had to chose between Chloride and Sulfate which one would be the better one?
All of chloride containing salts, that we might be interested in, <"are soluble">, which would be an advantage. The disadvantage is that chloride (Cl-) is monovalent, meaning that you need two of them when you add a divalent cation, so calcium chloride is CaCl2.nH2O etc.
Sulphates are a mixed bag in terms of solubility, but again it is only calcium sulphate (CaSO4.nH2O), that is both of interest to us and although not actually insoluble is "sparingly soluble".
For divalent cations sulphates are probably best, so magnesium sulphate (MgSO4.nH2O) rather than magnesium chloride (MgCl2).
The advantage of nitrate (potassium nitrate (KNO3 etc.)) and phosphate compounds (dipotassium phosphate (K2PO4) etc) would be both cation and anion are plant nutrients.
cheers Darrel
Last edited:
Thank you Darrel. The question was more to know if there was one worse than the other (for flora and fauna) in terms of concentration and if I had to prioritize one over the other, which one would it be.
I came up to the following theoretical remineralization strategy. I slightly changed my Ca:Mg ratio to 20:8 to bump up slightly dGH but also to have less comas in those numbers! I could go higher but don't think it's necessary. All shrimps have been doing just fine for the past year with that hardness. I think I better stay with MgSo4 + CaSo4. I could of course add some CaCl2 to further reduce sulfate but then I would have to deal with Cl and I am not sure of the benefits of this. There is probably already some Cl in the fertilizer I am using.
I want to add some MgCo3 to increase dKH as by week end I noticed the dKH is now close to 0. Alternatively I could also use CaCo3 which has the advantage of reducing overall PPM value by reducing SO4 PPM value. All this might sound a bit too simplistic to all you chemist cowboys but keep in mind I am trying to make this as simple as possible without the need to making complicated mixes while making things work.
1. Is there any drawback to this strategy and if I had to choose between MgCO3 and CaCo3, which one would be preferable?


2. I don't have the space to store large amounts of water and I normally throw in the MgSO4 and CaSo4 in the tank (in the skimmer - nice snow effect) straight after water change . This is usually not the standard polite procedure to remineralize water but since it's only dGH I figured it's not a big of a deal, at least I haven't seen any ill effect so far. However since I would now be adding carbonates to the water via MgCo3 or CaCo3, adding those directly to the tank would probably be a very bad (impolite, even deadly) idea due to PH possibly spiking. I know most people prepare they remineralized water and let it sit for a day or so. No can do. What are my options here?
Thank you gentlemen and gentlewomen.
I came up to the following theoretical remineralization strategy. I slightly changed my Ca:Mg ratio to 20:8 to bump up slightly dGH but also to have less comas in those numbers! I could go higher but don't think it's necessary. All shrimps have been doing just fine for the past year with that hardness. I think I better stay with MgSo4 + CaSo4. I could of course add some CaCl2 to further reduce sulfate but then I would have to deal with Cl and I am not sure of the benefits of this. There is probably already some Cl in the fertilizer I am using.
I want to add some MgCo3 to increase dKH as by week end I noticed the dKH is now close to 0. Alternatively I could also use CaCo3 which has the advantage of reducing overall PPM value by reducing SO4 PPM value. All this might sound a bit too simplistic to all you chemist cowboys but keep in mind I am trying to make this as simple as possible without the need to making complicated mixes while making things work.
1. Is there any drawback to this strategy and if I had to choose between MgCO3 and CaCo3, which one would be preferable?


2. I don't have the space to store large amounts of water and I normally throw in the MgSO4 and CaSo4 in the tank (in the skimmer - nice snow effect) straight after water change . This is usually not the standard polite procedure to remineralize water but since it's only dGH I figured it's not a big of a deal, at least I haven't seen any ill effect so far. However since I would now be adding carbonates to the water via MgCo3 or CaCo3, adding those directly to the tank would probably be a very bad (impolite, even deadly) idea due to PH possibly spiking. I know most people prepare they remineralized water and let it sit for a day or so. No can do. What are my options here?
Thank you gentlemen and gentlewomen.
Last edited:
Hi all,
cheers Darrel
I'm not sure there is a huge amount of difference, they would both be <"common anions"> in nearly all freshwater. Sulphate ions (SO4--) will contribute slightly more to the conductivity than chloride (Cl-) ions, purely because <"they have more charge">.The question was more to know if there was one worse than the other (for flora and fauna) in terms of concentration and if I had to prioritize one over the other, which one would it be.
I would just carry on, I think "shrimps have been doing just fine" is probably the only relevant bit.I slightly changed my Ca:Mg ratio to 20:8 to bump up slightly dGH ....... I could go higher but don't think it's necessary. All shrimps have been doing just fine for the past year with that hardness. I think I better stay with MgSo4 + CaSo4.
I think in practical terms there isn't any difference, CaCO3 is cheaper and easier to find, but neither is very soluble.Is there any drawback to this strategy and if I had to choose between MgCO3 and CaCo3, which one would be preferable?
The pH won't spike, it will just go to pH8 fairly slowly, calcium and magnesium carbonates are "weak bases". If you added a <"strong base"> (like sodium hydroxide (NaOH)) you would get a pH spike, potentially to pH11, because the NaOH would disassociate fully (and pretty much instantly) to Na+ and OH- ions.However since I would now be adding carbonates to the water via MgCo3 or CaCo3, adding those directly to the tank would probably be a very bad (impolite, even deadly) idea due to PH possibly spiking.
cheers Darrel
Thank you sir. As always, excellent.
MgCO3 is ~10 times more soluble than CaCO3, but yes comparatively to all compounds we have added they are highly insoluble.I think in practical terms there isn't any difference, CaCO3 is cheaper and easier to find, but neither is very soluble.
Very good news. Would there be a better time to add MgCO3? Say before CO2 is on, during or after, or it wouldn't matter?The pH won't spike, it will just go to pH8 fairly slowly, calcium and magnesium carbonates are "weak bases". If you added a <"strong base"> (like sodium hydroxide (NaOH)) you would get a pH spike, potentially to pH11, because the NaOH would disassociate fully (and pretty much instantly) to Na+ and OH- ions.
Hi all,
cheers Darrel
It is, but still less than 1/4 gram in a litre. Where this is relevant if you add both of them, Ca++ ions will <"come out of solution first"> (as CaCO3), before any MgCO3 forms, due to the <"common ion effect">.MgCO3 is ~10 times more soluble than CaCO3
When the CO2 was on would give you quicker dissolution, because you would have changed the <"CO2 ~ HCO3- ~ pH equilibrium point">. When the CO2 went off any "extra" of carbonate would <"come back out of solution"> as the <"least soluble"> carbonate salt, so realistically always as CaCO3.Say before CO2 is on, during or after, or it wouldn't matter?
cheers Darrel
You will get the best dissolution of CaCO3 when the water is most acidic, this usually means end of the day after CO2 injection. The faster you dissolve that CaCO3 the faster the pH spikes as the extra CO3 (2-) will bind with the available H+ and shift the equilibrium to the right.
As our aquariums are essentially open air(CO2)- CaCO3- H2O systems, in small amounts CO3 will eventually end up as HCO3 (distribution of carbonate species in relation to ph), reason why I sugest adding bicarbonates directly and avoid the entire pH spike and dissolution issues.
As our aquariums are essentially open air(CO2)- CaCO3- H2O systems, in small amounts CO3 will eventually end up as HCO3 (distribution of carbonate species in relation to ph), reason why I sugest adding bicarbonates directly and avoid the entire pH spike and dissolution issues.
Not sure I understood this.reason why I sugest adding bicarbonates directly and avoid the entire pH spike and dissolution issues.
Adding bicarbonates directly will not increase the pH as much or as abruptly and are readily soluble compared to CaCO3 . As you don't want to have a high pH and will ultimately end up with HCO3 in both cases, it makes sense to use bicarbonates from the start.
Hi all,
cheers Darrel
You only really have the option of potassium or sodium bicarbonate (KHCO3 / NaHCO3). It is only group 1. alkali metals that form soluble carbonates.Ok but which bicarbonate? ...adding potassium bicarbonate as I am already providing enough K through fertilizers.
cheers Darrel
Last edited:
X3NiTH
Member
- Joined
- 13 Apr 2014
- Messages
- 1,669
The reason I use pressurised CO2 into low temperature water via a Sodastream is to form as much Calcium Bicarbonate and Magnesium Bicarbonate as possible to aid in quick dissolution while adding extra Ascorbic acid helps further. The process really isn’t difficult or that technical it’s just a little bit labor intensive but it is better than adding a teaspoon of something you are wanting to limit.
If you really want complete control over exacting mineralisation then you have to get a little creative. You don’t have to use Sodastream, a Soda Bottle that uses the small disposable one time use cartridges would be sufficient.
🙂
If you really want complete control over exacting mineralisation then you have to get a little creative. You don’t have to use Sodastream, a Soda Bottle that uses the small disposable one time use cartridges would be sufficient.
🙂
Well it does require one to understand the chemical reactions. Personally I wouldn't dare go through that procedure as I am pretty certain I would screw things over. If you ever come to Thailand, let me know and I'll open my kitchen for a demonstration 🙂The process really isn’t difficult or that technical it’s just a little bit labor intensive