Paulo Soares
Member
- Joined
- 6 Nov 2014
- Messages
- 602
Good afternoon,
I´m not going to be very extensive cause i assume most of us know the "EI" assumptions and guideness.
For those who doens´t take a look here:
http://www.ukaps.org/index.php?page=dosing-with-dry-salts
So if we have a maximum plant Uptake of:
Nitrate (NO3) 20ppm per week
Phosphate (PO4) 3ppm per week
Please can anyone be kind to explain how a tank can consume at list 3 PPM of PO4 and more than 10 PPM of Nitrate a day?
Quite astonishing isn´t it?
(Be advised is not a testing issue)
Take this Ei receipe for an example.
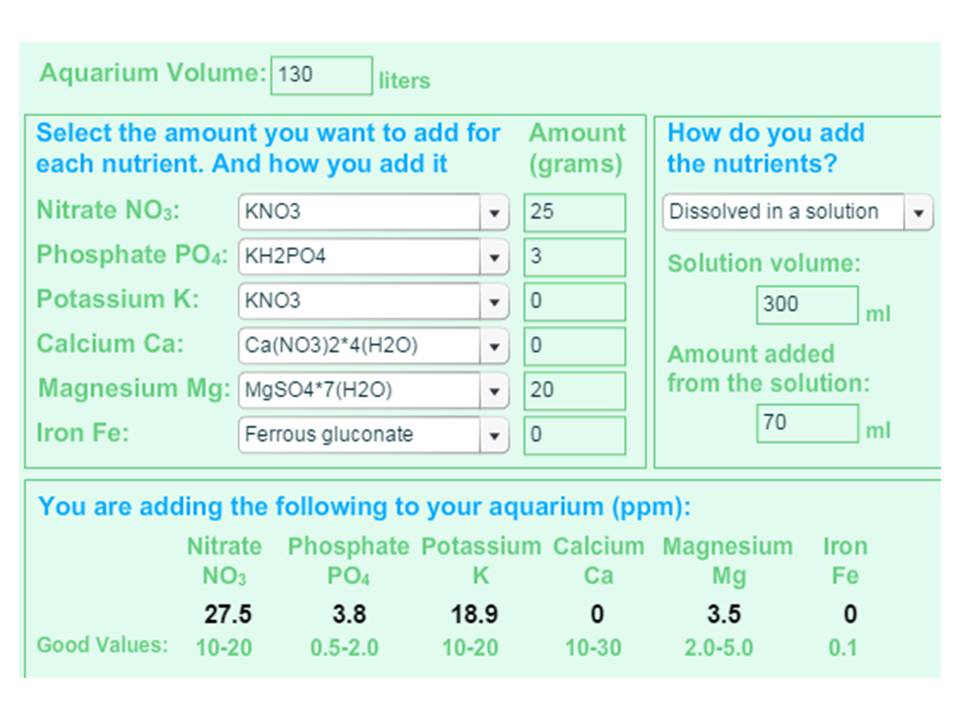
Could be any salt precipitation?
Would like to ear some opinions.
Thanks in advance.
I´m not going to be very extensive cause i assume most of us know the "EI" assumptions and guideness.
For those who doens´t take a look here:
http://www.ukaps.org/index.php?page=dosing-with-dry-salts
So if we have a maximum plant Uptake of:
Nitrate (NO3) 20ppm per week
Phosphate (PO4) 3ppm per week
Please can anyone be kind to explain how a tank can consume at list 3 PPM of PO4 and more than 10 PPM of Nitrate a day?
Quite astonishing isn´t it?
(Be advised is not a testing issue)
Take this Ei receipe for an example.
Could be any salt precipitation?
Would like to ear some opinions.
Thanks in advance.


