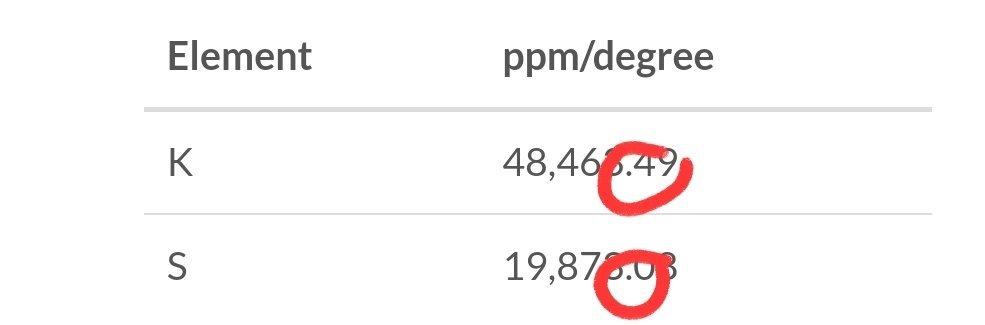
Hi all,
Ok, I understand that the hardness of water is important. Ideally it should be 6 or lower(?)
My tap water has an KH of 11. ICP analysis of water supplier tells me it contains Ca 56 ppm, Mg 6 ppm and K 2.6 ppm.
When i want to keep using this tap water as is....
To which ppm values should i dose Mg and K with these Ca and hardness numbers?
I understand that the ratio between those three is important. But is it wise to increase the Mg and K values to meet the ratios with the high Ca or should i keep them lower?
Or is it really worth to invest in RO unit? And all the hassle coming with it.
Thanks in advance,
Greetings Sandor
Ok, I understand that the hardness of water is important. Ideally it should be 6 or lower(?)
My tap water has an KH of 11. ICP analysis of water supplier tells me it contains Ca 56 ppm, Mg 6 ppm and K 2.6 ppm.
When i want to keep using this tap water as is....
To which ppm values should i dose Mg and K with these Ca and hardness numbers?
I understand that the ratio between those three is important. But is it wise to increase the Mg and K values to meet the ratios with the high Ca or should i keep them lower?
Or is it really worth to invest in RO unit? And all the hassle coming with it.
Thanks in advance,
Greetings Sandor