Yet the author expects us to not doubt that inaccurate data. Hopefully it's an exception and not common way of doing scientific research because if overall state of todays science would be based on such "valuable" data, we would be doomed as a humanity.Thanks for checking @Hanuman. Personally, I'm disappointed to read that an author is publishing data without being able to verify its accuracy.
-
You are viewing the forum as a Guest, please login (you can use your Facebook, Twitter, Google or Microsoft account to login) or register using this link: Log in or Sign Up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Long term effects of co2 exposure
- Thread starter Soilwork
- Start date
Hi all,
It is back to the <"bouncy castle"> analogy. Although CO2 is <"incredibly soluble">, at those sorts of CO2 levels you are literally going to have to be pumping CO2 in, because it will be lost at a great rate at the gas exchange surface. As you have more dissolved CO2 the quicker it will out-gas, because the CO2 equilibrium value at 415 ppm atmospheric CO2 is less than 3 mg / L.
I'll see what I can find in the scientific literature. I'll try and convert all the values from micro moles and <"partial pressure"> back to mg / L (ppm).
cheers Darrel
They definitely aren't right. edit: they <"maybe right">Yet the author expects us to not doubt that inaccurate data.
It is back to the <"bouncy castle"> analogy. Although CO2 is <"incredibly soluble">, at those sorts of CO2 levels you are literally going to have to be pumping CO2 in, because it will be lost at a great rate at the gas exchange surface. As you have more dissolved CO2 the quicker it will out-gas, because the CO2 equilibrium value at 415 ppm atmospheric CO2 is less than 3 mg / L.
I can understand why you might have elevated CO2 levels in still black-water habitats, but If you have calcium (Ca), CO2 and carbonate (CO3--) rich water, coming from a karst spring, all that will happen is that CaCO3 will precipitate out as soon as the water isn't under pressure. We get a <"lot of tufa formed locally"> where exactly that happens. Fast flow will also enlarge the gas exchange surface, so that is going to lower levels, it is the same as some-one having an air-stone come on to out-gas their tank after lights out.......... yes, indeed CO2 values can be sometimes very high in natural waters, for example in spring areas (Thailand), very fast flowing water, and black water biotopes.......
I'll see what I can find in the scientific literature. I'll try and convert all the values from micro moles and <"partial pressure"> back to mg / L (ppm).
cheers Darrel
Last edited:
Just to be clear, no one is putting into question your data.
I should have said. "I". Why you may ask? Because I am in no position to discredit data coming from someone with her knowledge. Not because she is God but because I have close to none compared to her. So I apologize to have included everyone with that statement.Why not? Questioning the results its the essence of science and research, while not questioning anything is an essence of faith and dogma.
This said you are correct, it's important to question as long as you are bringing something to the table. Else it's a moot point.
Last edited:
Hi all,
The conversion chart for partial pressure.
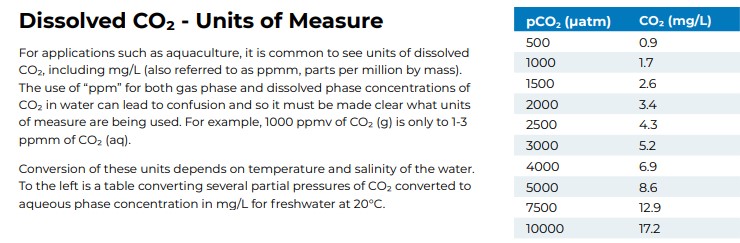
and here are some figures*:
So it looks like they aren't as unreasonable as values as I thought they were, but I'm still not entirely convinced that all the values are looking at dissolved CO2.
1 kPa ≈ 10,000 μatm ~1 mg / L and 20 kPa = 20 mg / L dissolved CO2? Differences in the powers of 10 (order of magnitude?)
*"Shartau, R. et al. (2022) "Acute CO2 tolerance in fishes is associated with air breathing but not the Root effect, red cell βNHE, or habitat",
Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 274." <"Acute CO2 tolerance in fishes is associated with air breathing but not the Root effect, red cell βNHE, or habitat">
cheers Darrel
The conversion chart for partial pressure.
and here are some figures*:
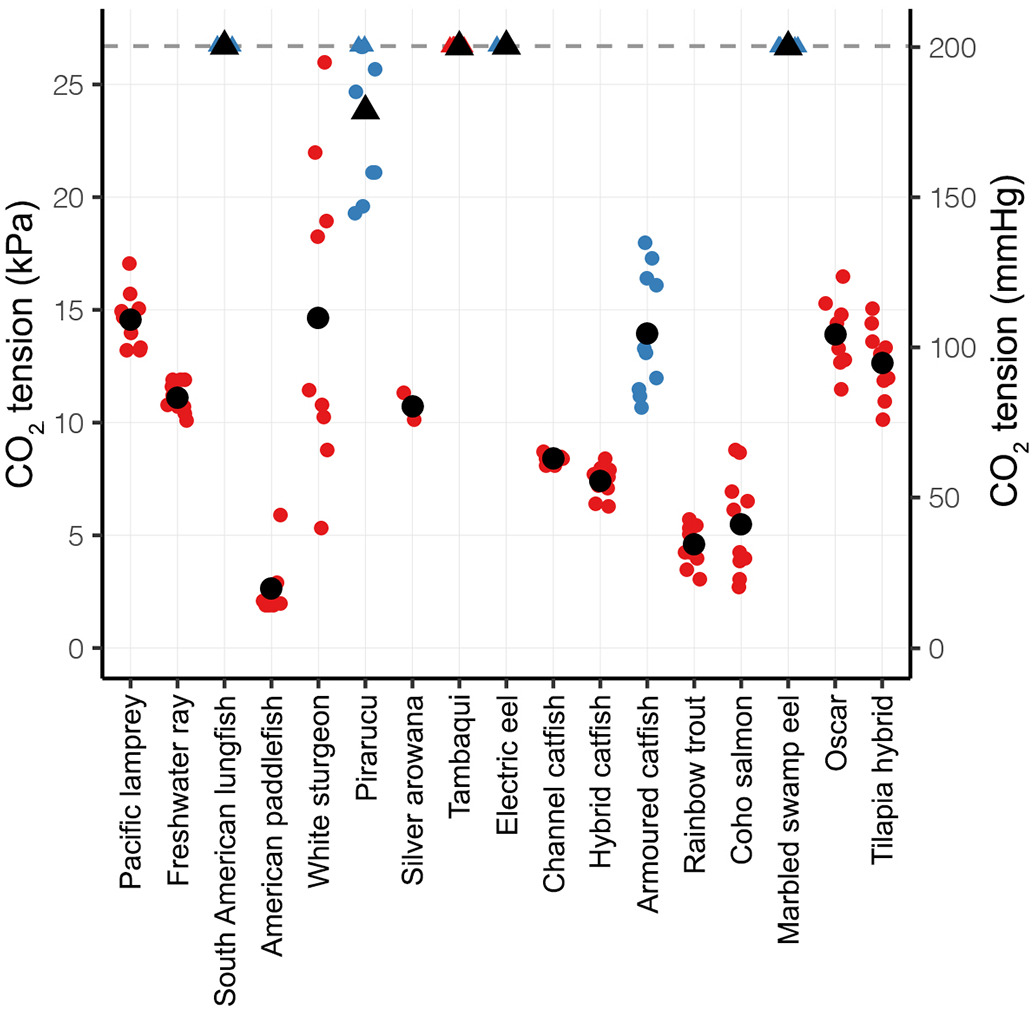
and a useful graph.......... Many aquatic environments naturally exhibit carbon dioxide partial pressures (PCO2) that greatly exceed the average global freshwater PCO2 value of ca. 0.3 kPa (ca. 3100 μatm; 1 kPa ≈ 10,000 μatm ≈ 1% ≈ 7.5 Torr ≈ 7.5 mmHg) (Cole et al., 1994; Hasler et al., 2016; Park et al., 1969; Raymond et al., 2013) due to a number of factors including microbial respiration, high biomass, presence of carbonate deposits, and the physicochemical properties of CO2 (Brauner et al., 2019). For example, PCO2 may reach values as high as 2–8 kPa in tropical systems (Cole et al., 1994; Li et al., 2013; de Rasera et al., 2013), 2–4 kPa in aquaculture ponds (Damsgaard et al., 2015), 0.5–2 kPa (10–40 mg L−1) in recirculating aquaculture systems (Brauner et al., 2019), 4.7 kPa (55 mg L−1) during fish transport (Allred et al., 2020), and 5–11 kPa (100–200 mg L−1) where CO2 is used as a chemical barrier against invasive species (Donaldson et al., 2016; Kates et al., 2012).......
So it looks like they aren't as unreasonable as values as I thought they were, but I'm still not entirely convinced that all the values are looking at dissolved CO2.
1 kPa ≈ 10,000 μatm ~1 mg / L and 20 kPa = 20 mg / L dissolved CO2? Differences in the powers of 10 (order of magnitude?)
*"Shartau, R. et al. (2022) "Acute CO2 tolerance in fishes is associated with air breathing but not the Root effect, red cell βNHE, or habitat",
Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 274." <"Acute CO2 tolerance in fishes is associated with air breathing but not the Root effect, red cell βNHE, or habitat">
cheers Darrel
Last edited:
Hi all,
It is still "as clear as mud" unfortunately, but I'm reverting to "unreasonable"
This is from "Hasler CT, Butman D, Jeffrey JD, Suski CD. (2016) Freshwater biota and rising pCO2? Ecol Lett. 19(1) pp 98-108. <"https://onlinelibrary.wiley.com/doi/10.1111/ele.12549">.
cheers Darrel
It is still "as clear as mud" unfortunately, but I'm reverting to "unreasonable"
This is from "Hasler CT, Butman D, Jeffrey JD, Suski CD. (2016) Freshwater biota and rising pCO2? Ecol Lett. 19(1) pp 98-108. <"https://onlinelibrary.wiley.com/doi/10.1111/ele.12549">.
Which at least tallies with my bouncy castle analogy and <"makes me feel better">, and then some (much smaller) numbers:......For instance, the first order streams with high terrestrial respiration have high levels of dissolved CO2 due to root respiration, however, should systems be in a calcium-rich landscape (e.g. limestone dominated regions), CO2 is quickly transformed into calcium bicarbonate. Should similar first order systems be in a non-calcium-dominated landscape, but are highly turbulent, much of the dissolved CO2 will quickly outgas. Furthermore, the pCO2 in small streams has been shown to be more closely linked to sources of groundwater, and as a watershed increases in size, the importance of subsurface flow towards supporting elevated CO2 concentrations decreases (Hotchkiss et al. 2015). Essentially, there are several factors that can be considered when making predictions about the amount of CO2 present in a freshwater system .......
....... A study of global freshwater lakes found that pCO2 ranged from 3.1-fold below to 16-fold above atmospheric pCO2, with a mean of ~ 1000 μatm – almost three times the current atmospheric level (Cole et al. 2007). Larger lakes tend to be closer to equilibrium with atmospheric CO2 (~ 390 μatm) due to the long residence time and outgassing of CO2, as, for example, the annual mean pCO2 measured in Lake Superior was 461 ± 171 μatm (mean ± standard deviation, SD) (Atilla et al. 2011) and in Lake Michigan values ranged from 250–500 μatm (Pilcher et al. 2015). In contrast, pCO2 levels for lotic systems can vary widely depending on stream order, forest cover, precipitation and surface area (Butman & Raymond 2011; Kokic et al. 2015); lotic systems can also become highly supersaturated with CO2, reaching partial pressures 10–15 times higher than atmospheric levels (Amazon River – Richey et al. 1980; Ottawa River – Telmer & Veizer 1999). Of the 6708 stream and river systems assessed at a global scale, 95% had a median pCO2 level greater than atmospheric levels, and the average pCO2 was found to be ~ 3100 μatm, which is almost 8-fold above current atmospheric levels (Raymond et al. 2013).......
Even if we go with the "15 times higher " that is ~6100 μ atm and only ~ 10 mg / L (ppm) dissolved CO2.........lotic systems can also become highly supersaturated with CO2, reaching partial pressures 10–15 times higher than atmospheric levels (Amazon River – Richey et al. 1980; Ottawa River – Telmer & Veizer 1999).........
cheers Darrel
Last edited:
Amano never really used high demanding plants, hence could get away with it. Some plants just do best with high CO2, most don't really care.Allegedly 15 ppm. Factually?
_Maq_
Member
Thank you for correction. You're right, I was wrong to link CO2 (half-)saturation to growth, in fact it is linked to photosynthesis. My apology.The half-saturation constant is not related to growth but to the nutrient uptake rate.
Just out of curiosity, could you kindly name some species which in your opinion demand truly high (>15 mg/L) concentration of CO2?Amano never really used high demanding plants, hence could get away with it.
hypnogogia
Member
This is the key question, 'Need' vs. 'Grow even more quickly'.Just out of curiosity, could you kindly name some species which in your opinion demand truly high (>15 mg/L) concentration of CO2?
_Maq_
Member
You've touched by chance the core of my theory/opinion on CO2 injection:This is the key question, 'Need' vs. 'Grow even more quickly'.
Plants grow (=create new tissues, leaves) and die (=drop older leaves) always simultaneously. In low-tech treatment, we expend our knowledge and skills to slow-down dying. In hi-tech treatment, we expend money (=CO2 gear and hi-tech lighting) to speed-up growing, and become less dependent on our knowledge and skills to slow-down dying.
Me, as a low-tech die-hard, if I make even a minor mistake, it takes months until plants in question take their healthy shape again. It's frustrating yet at the same time challenging and stimulating.
If it is the half-saturation constant for CO2 it links for example mmol CO2 m-3 to umol C g-1 h-1. If it‘s the half-saturation constant for PO4 it links mmol PO4-P m-3 (water concentration) to umol P g-1 h-1 (uptake rate). For an example of that last one , see the paper of the famous Jensen, Andersen and Christiansen. It‘s always environmental availability linked to the plant, animal whatever uptake/use of the substrate.Thank you for correction. You're right, I was wrong to link CO2 (half-)saturation to growth, in fact it is linked to photosynthesis. My apology.
Personally I don‘t desire to setup my tanks with the goal of creating an environment where only the minimum requirements are being met and creatures barely hang on to life. I‘m setting something to enjoy, not the stage for the next Bear Grylls episode. Just because my fish can survive at 3 mg/L oxygen, doesn‘t mean that I purposefully reduce the oxygen concentration in the tank to that value. I aim to be well higher than that at all times actually, more than covers their basic needs. By analogy, maybe in your eyes that makes me a less knowledgeable/skillful fish keeper. That‘s fine, my fish and plants will benefit from what little abundance I can afford them.
Last edited:
Hi all,
cheers Darrel
Should that be three mg / L CO2? or are you saying that you aim for more than three mg / L dissolved oxygen, even though your fish will survive at that level?I don‘t aim to have no more than 3 mg/L oxygen in the aquarium, because my fish can deal with it
cheers Darrel
Last edited:
_Maq_
Member
Very well, but as I know, you observe my current experiment. There, you'll be in difficulty to point to any plant benefiting from extra feeding compared to tank D - lean. Would you say, then, that those plants "barely hang on to life"?Personally I don‘t desire to setup my tanks with the goal of creating an environment where only the minimum requirements are being met and creatures barely hang on to life. I‘m setting something to enjoy
Darrel, I edited the previous post for clarity. It was O2.
Maq, as I recall, the tanks in your journal had neither fish nor CO2 injection so I don‘t see how they are relevant examples of the subject in this thread. Let’s put it as ... some plants look better than others. It’s your tank, if the goal you had when setting it up was that look, and you achieved it in one of the tanks, then great. Enjoy it.
It might be the case that other people, when they plan for a tank in their living room, desire a tank that looks more similar to Greggz‘ tank
 _
_
If as you suggest, a tank looking like this is the fruit of lacking knowledge and skill to ‘expend‘, plants and fish sure seem to benefit from their approach for quite a number of years now. Injecting CO2 at 30 ppm and keeping aquarium fish seems doable.
Maq, as I recall, the tanks in your journal had neither fish nor CO2 injection so I don‘t see how they are relevant examples of the subject in this thread. Let’s put it as ... some plants look better than others. It’s your tank, if the goal you had when setting it up was that look, and you achieved it in one of the tanks, then great. Enjoy it.
It might be the case that other people, when they plan for a tank in their living room, desire a tank that looks more similar to Greggz‘ tank
If as you suggest, a tank looking like this is the fruit of lacking knowledge and skill to ‘expend‘, plants and fish sure seem to benefit from their approach for quite a number of years now. Injecting CO2 at 30 ppm and keeping aquarium fish seems doable.
- Joined
- 22 Nov 2015
- Messages
- 559
Hi All,
Just for arguments sake and actually to hopefully prevent a co2 vs non co2 argument ensuing. The reason I created this thread was because a poster on another forum said that if certain plants cannot grow underwater without supplemental co2 then they shouldn’t be under water and then went on to say how co2 was harmful to fish and inverts etc.
Now I’m not a co2 user but I used to be and would probably use it again in the future and I know that it is not correct to say that co2 harms livestock without knowing what levels we are talking about. I did reference the table above as evidence to suggest that natural waters can be high in co2 but didn’t realise that these values were calculated and not measured.
Given that my thoughts on aquariums is similar to that of Darrel and most of UKAPS I’m sure by now is that oxygen is the most important parameter of concern. If we believe this to be true and we have witnessed pearling in our co2 injected tanks then we are practicing our mantra to the extreme. So I definitely see the value in co2 for plants. It’s the livestock I am concerned about.
Just for arguments sake and actually to hopefully prevent a co2 vs non co2 argument ensuing. The reason I created this thread was because a poster on another forum said that if certain plants cannot grow underwater without supplemental co2 then they shouldn’t be under water and then went on to say how co2 was harmful to fish and inverts etc.
Now I’m not a co2 user but I used to be and would probably use it again in the future and I know that it is not correct to say that co2 harms livestock without knowing what levels we are talking about. I did reference the table above as evidence to suggest that natural waters can be high in co2 but didn’t realise that these values were calculated and not measured.
Given that my thoughts on aquariums is similar to that of Darrel and most of UKAPS I’m sure by now is that oxygen is the most important parameter of concern. If we believe this to be true and we have witnessed pearling in our co2 injected tanks then we are practicing our mantra to the extreme. So I definitely see the value in co2 for plants. It’s the livestock I am concerned about.
Hi all,
cheers Darrel
Thank-you, it did make sense as "oxygen" the first time, but that is unambiguous.Darrel, I edited the previous post for clarity. It was O2.
I understand that, it isn't <"what I'm interested in">, but I have an <"understanding wife"> and she doesn't insist on <"aesthetic quality"> (and the tanks are in the kitchen). We have plenty of members with stunning high-tech tanks with <"healthy fish"> and <"plants">.It might be the case that other people, when they plan for a tank in their living room, desire a tank that looks more similar to Greggz‘ tank
cheers Darrel
Andy Pierce
Member
I think if you witness pearling in your CO2 injected tank it means you don't have enough water flow.If we believe this to be true and we have witnessed pearling in our co2 injected tanks then we are practicing our mantra to the extreme.
The poster's statement doesn't make sense unless those plants were developed from scratch (including their genetic modification etc. ) to survive only at artificially elevated levels of CO2. What happened to them before CO2 age in aquatic hobby? Were they even growing and reproducing in the nature before, being able to accept common levels of CO2 found in the rivers etc?a poster on another forum said that if certain plants cannot grow underwater without supplemental co2
Hi all,
It is the <"Murdannia keisak"> or <"wet paddy rice"> scenario.

These are usually plants that are <"amphibious">, rather than <"(obligate) aquatic"> - <"https://nph.onlinelibrary.wiley.com/doi/full/10.1111/nph.16347">.
cheers Darrel
No, I think it does make sense and that they are right........... certain plants cannot grow underwater without supplemental co2
I think it is much simpler than that, they weren't growing in rivers (or not permanently), they are plants that are perfectly adapted to <"atmospheric CO2 levels">.unless those plants were developed from scratch (including their genetic modification etc. ) to survive only at artificially elevated levels of CO2. What happened to them before CO2 age in aquatic hobby? Were they even growing and reproducing in the nature before, being able to accept common levels of CO2 found in the rivers
It is the <"Murdannia keisak"> or <"wet paddy rice"> scenario.
These are usually plants that are <"amphibious">, rather than <"(obligate) aquatic"> - <"https://nph.onlinelibrary.wiley.com/doi/full/10.1111/nph.16347">.
..... Amphibious plants thrive in areas with fluctuating water levels, partly as a result of their capacity to make specialized leaves when submerged or emerged. The tailor-made leaves improve gas exchange underwater or prevent aerial desiccation. Aquatic leaves are thin with narrow or dissected forms, thin cuticles and fewer stomata. These traits can combine with carbon-concentrating mechanisms and various inorganic carbon utilization strategies. Signalling networks underlying this plasticity include conserved players like abscisic acid and ethylene, but closer inspection reveals greater variation in regulatory behaviours. Moreover, it seems that amphibious leaf development overrides and reverses conserved signalling pathways of their terrestrial counterparts. ......
cheers Darrel
- Joined
- 22 Nov 2015
- Messages
- 559
Hi all,
No, I think it does make sense and that they are right.
I think it is much simpler than that, they weren't growing in rivers (or not permanently), they are plants that are perfectly adapted to <"atmospheric CO2 levels">.
It is the <"Murdannia keisak"> or <"wet paddy rice"> scenario.
View attachment 203660
These are usually plants that are <"amphibious">, rather than <"(obligate) aquatic"> - <"https://nph.onlinelibrary.wiley.com/doi/full/10.1111/nph.16347">.
cheers Darrel
I actually do agree with the statement as most plants we use are semi aquatic by nature. Those that grow well without injection show us that they can adapt to life underwater in an aquarium so it’s not true to say that these plants ‘need’ periods of atmospheric exposure in order to survive. Other plants or ‘high tech’ plants that don’t do very well without co2 injection you could definitely argue that they need prolonged periods of atmospheric exposure to truly thrive.
Its is these plants that you could argue do not share the same spatial requirements with our fauna from a natural perspective.
Hi all,

In the bottom of some of the <"turloughs you have Eleocharis acicularis">, it must spend years submerged before the "tide" eventually goes out and it flowers. <"https://onlinelibrary.wiley.com/doi/full/10.1002/eco.2316">
I've not tried <"Murdannia keisak">, but I'd be very interested if any-one has kept it underwater long term.
cheers Darrel
Yes, agreed. I've had Echinodorus bleheri, Anubias barteri, Cryptocoryne spp., <"Bolbitis heudelotii"> & Hygrophila corymbosa plants that have spent at least the last ten years submerged, and probably a lot longer. I'm guessing that is partially why these sorts of plants persist in the hobby, you can produce them emersed, but grow them submerged indefinitely.Those that grow well without injection show us that they can adapt to life underwater in an aquarium so it’s not true to say that these plants ‘need’ periods of atmospheric exposure in order to survive.
In the bottom of some of the <"turloughs you have Eleocharis acicularis">, it must spend years submerged before the "tide" eventually goes out and it flowers. <"https://onlinelibrary.wiley.com/doi/full/10.1002/eco.2316">
I've not tried <"Murdannia keisak">, but I'd be very interested if any-one has kept it underwater long term.
........ I've not grown <"Murdannia keisak">, but I have grown the very similar <"Commelina communis"> (for <"experimental purposes">) and once flowering is initiated nothing will stop it and it will flower and die. ......
cheers Darrel
Last edited:

