I would be careful with Steam Iron Water as it may have perfume in it!
De-ionsed water for cars will be okay!
De-ionsed water for cars will be okay!
But avoid battery top up fluid, as that may not be just deionised water (even if labelled as such) ... Could contain sulphuric acid!I would be careful with Steam Iron Water as it may have perfume in it!
De-ionsed water for cars will be okay!
avoid battery top up fluid, as that may not be just deionised water (even if labelled as such) ... Could contain sulphuric acid!
I didn't know that, I always just get a small amount from the <"lab. DI unit">. I don't know exactly how much DI water we get through in a week (for rinsing the glassware etc), but it will be hundreds of litres.I would be careful with Steam Iron Water as it may have perfume in it!
Distilled, <"DI or RO water is perfect">, it is the lack of ions that makes it suitable, it is just a blank slate to start off with. What we call "water" is really a weak solution of salts with H2O as the solvent. Because H2O is an <"amphoteric solvent"> a lot of substances will go into solution in it.I know this is an old post but wanted to check if De-Ionised Water the stuff they use in cars and irons could be used in my macro mix? Or will the lack of ions affect the salts.
In my case it is definitely Mn. It is quite interesting that I can even control plants growth rate by changing Mn dose (by defining Mn:Fe ratio in my micro dosing). Algae is also Mn defficient surprisingly (or not so surprisingly).


Unfortunately a bit of chemistry is inevitable. I'm not a chemist, I've found a lot of <"chemistry quite difficult to understand"> and I still only know the bits <"I've run into over time">.like a chemistry lecture
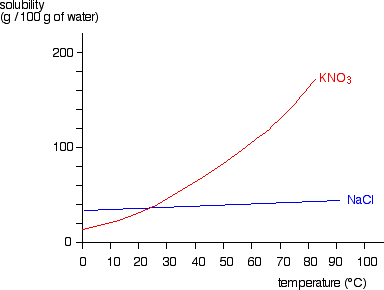
Yes, solubility increases for most salts with temperature, <"carbonates are the exception">. The problem is that as the water cools they tend to come back out of solution, the <"least soluble salt first">. You can increase carbonate solubility by reducing the temperature <"and/or adding CO2">, but the same applies, as the temperature rises, or CO2 escapes, the carbonates come back out of solution.Is it possible to use the microwave to heat the solution and increase the solubility ?
So it's a false good idea, i don't use any carbonate i think, chloride and sulfates mostly, is the microwave change salt reaction, It seems to me that the microwave makes the water molecules rub together to heat up ? won't alter the salt ? thxHi all,
Yes, solubility increases for most salts with temperature, <"carbonates are the exception">. The problem is that as the water cools they tend to come back out of solution, the <"least soluble salt first">. You can increase carbonate solubility by reducing the temperature <"and/or adding CO2">, but the same applies, as the temperature rises, or CO2 escapes, the carbonates come back out of solution.
When we make up stock solutions in the lab. we use a magnetic stirrer and hot plate, but you still can't exceed the total solubility limits.
cheers Darrel
It is literally <"just the heating">, it doesn't matter how you heat the water.It seems to me that the microwave makes the water molecules rub together to heat up ? won't alter the salt ?
All chlorides (other than <"silver chloride (AgCl)"> are soluble.i think, chloride and sulfates mostly,
Hi @Vsevolod StakhovBtw, since potassium tests are not really available in the UK...

It gets a mention earlier in <"the thread">. It should work OK, other than the difficulty of saying exactly when the "X" has disappeared.I use the JBL potassium test kit.
Hi @dw1305It gets a mention earlier in <"the thread">. It should work OK, other than the difficulty of saying exactly when the "X" has disappeared.
Hi @eminor, Yes, if indeed the storage bottle delivers 2ppm per 1.6 ml to your tank and you transfer 100 ml from the storage bottle to a 100 ml pump bottle it will deliver the same 2 ppm. per 1.6 ml. pump... Do shake the storage bottle well before you transfer to the pump bottle.It might be a dumb question but to be sure, my 500 ml K2SO4 + H2O i made is my storage backup bottle. I made that mix to deliver 2 ppm K every 1.6 ml. My little pump 100ml deliver 1.6 ml. So if i'm right as long as i don't add anaything, the ppm never change ? i mean if i take 100 ml from the storage bottle and put it to the pump bottle, the 1.6 ml will still deliver 2ppm K ? thx
