Thanks everyone! It's still a mistery, but the deaths slowed down at least. I'll finish the interpet treatment then maybe do a round of ndx after it clears and hope for the best. I wanted to move the remaining chilli rasboras, some shrimp and some plants into my new bowl scape, but this makes me quite reluctant, don't want to take any disease over there too. Any ideas if it would transfer?
Also got rid of the blood worms.
Probably done with these pseudomugil rainbows, they have so many issues - they jump, they need a night light, prone to disease, they even choke on food all the time and have to be rescued.
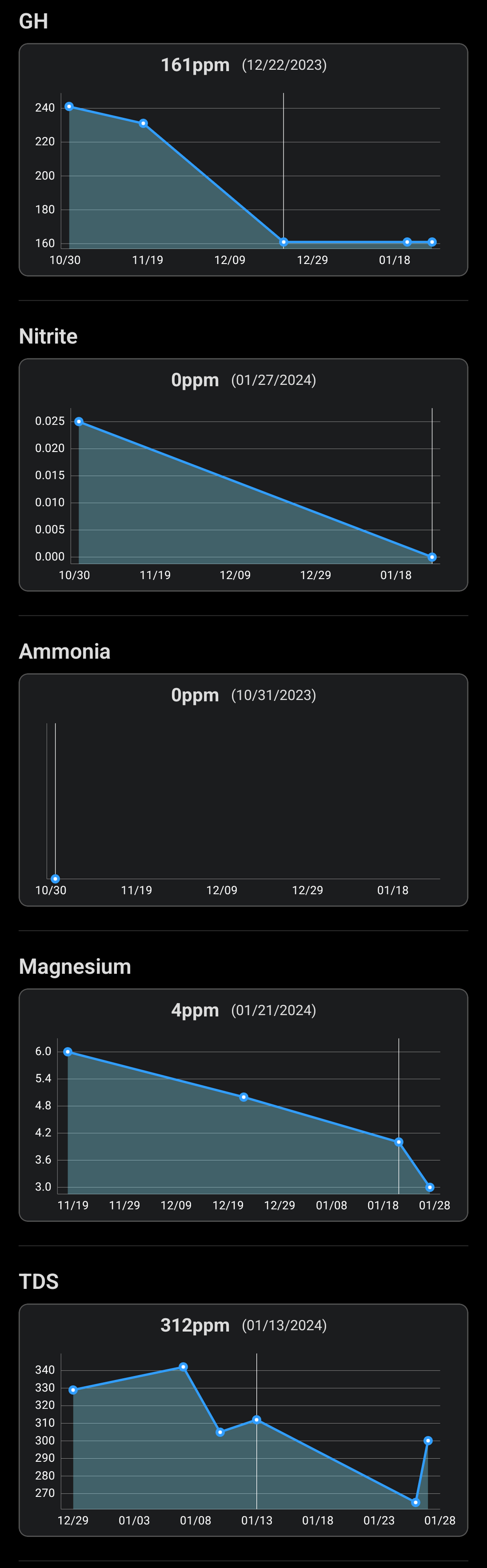
Regarding the filter media, I rinsed the sponges today and the flow increased. I was actually thinking of removing some of the ceramics or maybe all to give more space to the roots. Pretty sure the plants take up all the ammonia before the bacteria can do much. Even have the seachem ammonia alert, but never saw it budge from 0.
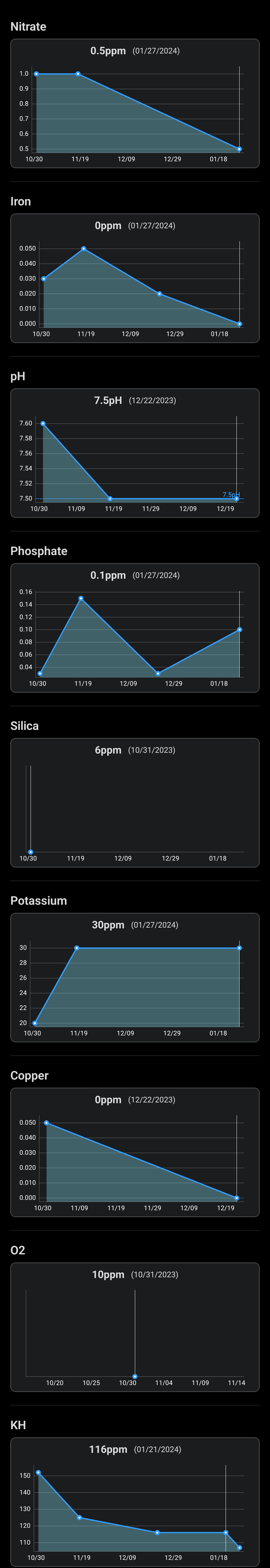
The nitrates - I'm sure there's some, especially as I'm dosing them, but it must be below 1ppm. The plants are always just on the edge, if I stop they start getting holes and yellow leaves in a matter of days. The colour on the jbl test kit is always between the two lowest swatches, closer to the lowest one (so <1ppm),though as Darrel said, it's not a very good test.