-
You are viewing the forum as a Guest, please login (you can use your Facebook, Twitter, Google or Microsoft account to login) or register using this link: Log in or Sign Up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Consistency Deficiency
- Thread starter Hufsa
- Start date
@Wookii it is a black loofah, something like these:

I bought mine off ebay, I was only able to source the ones that look highlighted like the one on the right, this kind has two stripes of white running through it but I use just the black parts, works fine unless you need to cover a very large area. I place the mesh from above and then twist the loose ends around to a point under the rock/coconut. It helps to take the moss by surprise as it can sense hesitation and it gets messy if you move the mesh on top around after you have started. I then finish up with a small black zip tie and cut off the mesh that sticks out.
Please be warned though if you use it on something hollow under like a coconut, its risky to certain kinds of fish, they can get stuck in there, I lost a kuhli from it poor thing. The safest is to use stone where there are no gaps between the mesh and the stone.

I bought mine off ebay, I was only able to source the ones that look highlighted like the one on the right, this kind has two stripes of white running through it but I use just the black parts, works fine unless you need to cover a very large area. I place the mesh from above and then twist the loose ends around to a point under the rock/coconut. It helps to take the moss by surprise as it can sense hesitation and it gets messy if you move the mesh on top around after you have started. I then finish up with a small black zip tie and cut off the mesh that sticks out.
Please be warned though if you use it on something hollow under like a coconut, its risky to certain kinds of fish, they can get stuck in there, I lost a kuhli from it poor thing. The safest is to use stone where there are no gaps between the mesh and the stone.
@Wookii it is a black loofah, something like these:
View attachment 184244
I bought mine off ebay, I was only able to source the ones that look highlighted like the one on the right, this kind has two stripes of white running through it but I use just the black parts, works fine unless you need to cover a very large area. I place the mesh from above and then twist the loose ends around to a point under the rock/coconut. It helps to take the moss by surprise as it can sense hesitation and it gets messy if you move the mesh on top around after you have started. I then finish up with a small black zip tie and cut off the mesh that sticks out.
Please be warned though if you use it on something hollow under like a coconut, its risky to certain kinds of fish, they can get stuck in there, I lost a kuhli from it poor thing. The safest is to use stone where there are no gaps between the mesh and the stone.
Great stuff! Do you find it lasts well in the tank (i.e. doesn’t disintegrate over time)?
If my method isnt quite clear I can do a little picture tutorial once I am feeling better, I am still pretty sick and I know you guys wouldn't want me to rush with it so it would have to be in a few days. Otherwise as an alternative I think I originally learned it from a Rachel O'Leary youtube video, if someone can find it feel free to post it here.
Thats a good question about longevity @Wookii , Ive never had a problem with it, but since I suffer from chronic consistency deficiency I do change up my mossy rocks and whatnots every now and then. But the mesh ones with susswassertang have been in there for at least a year now it must have been, maybe year and a half. It seems exactly the same as when I put it in
What'cha planning to wrap? 😃
Thats a good question about longevity @Wookii , Ive never had a problem with it, but since I suffer from chronic consistency deficiency I do change up my mossy rocks and whatnots every now and then. But the mesh ones with susswassertang have been in there for at least a year now it must have been, maybe year and a half. It seems exactly the same as when I put it in
What'cha planning to wrap? 😃
Im really not feeling well as I am writing this, so the update is a bit short and unedited. Still dealing with the aftermath of the virus coupled with making some existing health issues worse, so it is what it is. The pictures are uncropped and probably not the best.
Firstly the happiest news I have had in a while, exactly a week ago I was watching the tank when I suddenly saw a silvery flash go by in a little hole in the wood. The Long Serpent lives! The cheeky little monkey had me thinking he was 6 feet under, pushing up daisies and pining for the fjords, that I had somehow missed finding the body and the rest of the inmates had disposed of the evidence. But no, this sneaky little snake has been hanging out in his own personal clubhouse having a jolly good time, leaving me worrying all week for nothing!

He was eventually convinced to come out using some earthworm sticks as bait, although it took him at least ten minutes of sniffing the water, throwing his head to and fro in anger that he had to leave his cosy little cubbyhole to get at the food, and then some more pined considerations until he finally came tumbling out.

Ive since seen him out and about a few times, but he appears to spend most of his time in the wood. He seems to have memorized an easy way of getting in and out, as I can be looking at him one moment, turn my head and the next he is gone and will eventually appear in his little window. The majority of the wood is hollow, so I think he is entering from the end of the branch and slithering his way up somehow.



He came out again a few days ago to have a bit of a smorgasbord with his friends. I wonder if an assembly of noodles can be called a spaghetti.

FTS. Plants are meh. Mixed bag.

The tangle in the middle is a second crypt "rescued" from the LFS, if all goes to plan this one and the existing one to the left are "Silver Queen". Right now they're not looking much like anything, so only time will tell.



Unhappy Tonina and Pantanal, Pantanal is doing some stunting looking business and the Tonina just looks pale. Cuba is still a bit pale but growing pretty well and increasing in size unlike its poor brother.


Rotalas not showing much improvement that I can see. I havent changed any of the ferts from last I wrote, ive just been too sick.
Cryptocoryne purpurea seems happy and is sending out increasingly larger new leaves. C. fusca "Queen Vandom" is sending out a new fat looking leaf as well. At least I can grow something 😕 Narrow brownish looking leaves to the right of it is Cryptocoryne ferruginea I picked up at the same time as the second Silver Queen.



Ammannia pedicellata "Golden" has stunted real good. I wonder if the increase in ferts did it, or if it was something else entirely. Not enough data to conclude anything. Ammannia crassicaulis or whatever the correct name is seems ok enough, a few stunted stems but the ones that are growing look good. Both Pogostemon and Guyana seem just fine.
I havent gotten around to a water change yet, its half a week overdue. I ran out of the premade remineralizer and my brain has just been too foggy to work out how much CaSO4 and MgSO4 to add to the water change barrel from the seperate powders I have. Hope to have it done by the weekend
Will leave you with this picture I have titled "Existence is pain - food too far away"

Firstly the happiest news I have had in a while, exactly a week ago I was watching the tank when I suddenly saw a silvery flash go by in a little hole in the wood. The Long Serpent lives! The cheeky little monkey had me thinking he was 6 feet under, pushing up daisies and pining for the fjords, that I had somehow missed finding the body and the rest of the inmates had disposed of the evidence. But no, this sneaky little snake has been hanging out in his own personal clubhouse having a jolly good time, leaving me worrying all week for nothing!

He was eventually convinced to come out using some earthworm sticks as bait, although it took him at least ten minutes of sniffing the water, throwing his head to and fro in anger that he had to leave his cosy little cubbyhole to get at the food, and then some more pined considerations until he finally came tumbling out.

Ive since seen him out and about a few times, but he appears to spend most of his time in the wood. He seems to have memorized an easy way of getting in and out, as I can be looking at him one moment, turn my head and the next he is gone and will eventually appear in his little window. The majority of the wood is hollow, so I think he is entering from the end of the branch and slithering his way up somehow.



He came out again a few days ago to have a bit of a smorgasbord with his friends. I wonder if an assembly of noodles can be called a spaghetti.

FTS. Plants are meh. Mixed bag.

The tangle in the middle is a second crypt "rescued" from the LFS, if all goes to plan this one and the existing one to the left are "Silver Queen". Right now they're not looking much like anything, so only time will tell.



Unhappy Tonina and Pantanal, Pantanal is doing some stunting looking business and the Tonina just looks pale. Cuba is still a bit pale but growing pretty well and increasing in size unlike its poor brother.


Rotalas not showing much improvement that I can see. I havent changed any of the ferts from last I wrote, ive just been too sick.
Cryptocoryne purpurea seems happy and is sending out increasingly larger new leaves. C. fusca "Queen Vandom" is sending out a new fat looking leaf as well. At least I can grow something 😕 Narrow brownish looking leaves to the right of it is Cryptocoryne ferruginea I picked up at the same time as the second Silver Queen.



Ammannia pedicellata "Golden" has stunted real good. I wonder if the increase in ferts did it, or if it was something else entirely. Not enough data to conclude anything. Ammannia crassicaulis or whatever the correct name is seems ok enough, a few stunted stems but the ones that are growing look good. Both Pogostemon and Guyana seem just fine.
I havent gotten around to a water change yet, its half a week overdue. I ran out of the premade remineralizer and my brain has just been too foggy to work out how much CaSO4 and MgSO4 to add to the water change barrel from the seperate powders I have. Hope to have it done by the weekend
Will leave you with this picture I have titled "Existence is pain - food too far away"

John q
Member
MichaelJ
Member
Hey @Hufsa - my favorite Mummitroll !! Get well soon!Im really not feeling well as I am writing this, so the update is a bit short and unedited. Still dealing with the aftermath of the virus coupled with making some existing health issues worse, so it is what it is.
Nice pictures!
Best,
Michael
KirstyF
Member
Hope ur feeling better soon hon.
Lovin ur puddle of noodles. 😍
Lovin ur puddle of noodles. 😍
love the variety of plants. I have found it hard for Pantanal and A.Pedicatella to co-exist though. The former loves EI dosing while the latter likes lean dosing.....
I guess the important thing now is to have consistency and see which plants thrive - those that thrive are keepers, while those that stunt badly can be replaced with something new.... just repeat the process until you have a tank full of plants that are happy with the ferts/water parameters
I guess the important thing now is to have consistency and see which plants thrive - those that thrive are keepers, while those that stunt badly can be replaced with something new.... just repeat the process until you have a tank full of plants that are happy with the ferts/water parameters
plantnoobdude
Member
if you look at happis pictures, you will see it;s possible. ammannia and pantanal thriving.love the variety of plants. I have found it hard for Pantanal and A.Pedicatella to co-exist though. The former loves EI dosing while the latter likes lean dosing.....
I guess the important thing now is to have consistency and see which plants thrive - those that thrive are keepers, while those that stunt badly can be replaced with something new.... just repeat the process until you have a tank full of plants that are happy with the ferts/water parameters
Happi-singh
Thanks for the wishes everyone 🥰

Right now on my to-do list:
-Scrunch brain enough to figure out how to remineralize with new salts, then do water change.
-When brain satisfactorily un-smoothed, redo micro mix.
Gotta fix whatever this chlorosis thing is. Whats bugging me is that the sheer amount of iron that is currently being added should be more than sufficient. Its being added daily so theoretically I should be able to use whatever chelate I want. To just keep blindly adding more of the same when its obviously not working quite right seems like a mistake somehow. I should google how manganese deficiency presents, maybe Happi is onto something with all that stuff. I want some more DTPA because that seems to have worked well in the past, and would like to go fully custom ratios while im at it but im not sure when I will trust myself to do that sort of detail work again. Mess up potassium in a mix and whoopsie, nothing will happen. Mess up copper more than just a little bit and whoopsie all your fish are dead. Stakes are bit higher. So more DTPA iron yes, custom traces maybe not quite yet.
My CO2 implementation is almost certainly bad right now, the drop checker seems to have taken a turn for the bluer, and without a proper ph profile the whole thing is just an unknown. I have quite aggressive growth of BBA and thread algae. I had to stop trimming off algae coated leaves on the buces, at the rate things were going I would have killed the plants. So I need to deal with the root cause before trimming off any more. I was never a fan of treating symptoms anyway, partly from principle and partly laziness. Ive been pretty good at manually removing hair algae recently, but it is competing with water changes for my very limited amount of energy, so im not sure if I am approaching that at the right end. I would think water changes are more important than manual removal of algae, but im not absolutely sure.
Thanks, its pathological 😁 ..Yeah I sorta knew Pantanal and Pedicellata might be a difficult pair of plants to match. Im a sucker for punishment though. Difficult is where all the good learn'dings are at, at least I think it is 🤔 I wasnt feeling too optimistic about things yesterday but thanks to the marvel of modern medicine I am feeling a bit less dead today and my outlook has increased somewhat to matchlove the variety of plants. I have found it hard for Pantanal and A.Pedicatella to co-exist though. The former loves EI dosing while the latter likes lean dosing.....
The only good thing that came from being so gosh darng sick was that I couldnt physically mess with the ferts no matter how much I wanted to, so I was just forced to let things run its course for a while. If I actually tally up the species that are doing good vs the ones doing poorly, it doesnt seem that bad at all. I am drawn to focus on the things that arent going well, so im going to keep trying to grow both out of good old fashioned stubbornness. From what I hear Pedicellata is at the higher end of the "prefers lean" scale, so it might be possible to grow both satisfactorily. Even if I ultimately fail I like to give things a good try. If I had to pick one of the two species to keep, I think I would have to fall on the side of pedicellata, the Golden just looks so nice when its happy, while you can get all manner of orange/red/pink from a large variety of other plants than Pantanal. I hear the one they call Meta is much easier to grow, probably wont be able to source that any time soon but is a good candidate for trying down the road.I guess the important thing now is to have consistency and see which plants thrive - those that thrive are keepers, while those that stunt badly can be replaced with something new.... just repeat the process until you have a tank full of plants that are happy with the ferts/water parameters
I took a look but couldnt immediately identify, it might be I am still a bit too smooth-brained post virus but the blurple tone on some of the pics didnt help me with the ID's 😅 Ive seen some of his pictures in the lean dosing thread, for a lot of them I wish they came with more in depth context, yes they're very pretty plants but I like really specific details, how, when, why, before/after, tell me everything.if you look at happis pictures, you will see it;s possible. ammannia and pantanal thriving.
Happi-singh
Right now on my to-do list:
-Scrunch brain enough to figure out how to remineralize with new salts, then do water change.
-When brain satisfactorily un-smoothed, redo micro mix.
Gotta fix whatever this chlorosis thing is. Whats bugging me is that the sheer amount of iron that is currently being added should be more than sufficient. Its being added daily so theoretically I should be able to use whatever chelate I want. To just keep blindly adding more of the same when its obviously not working quite right seems like a mistake somehow. I should google how manganese deficiency presents, maybe Happi is onto something with all that stuff. I want some more DTPA because that seems to have worked well in the past, and would like to go fully custom ratios while im at it but im not sure when I will trust myself to do that sort of detail work again. Mess up potassium in a mix and whoopsie, nothing will happen. Mess up copper more than just a little bit and whoopsie all your fish are dead. Stakes are bit higher. So more DTPA iron yes, custom traces maybe not quite yet.
My CO2 implementation is almost certainly bad right now, the drop checker seems to have taken a turn for the bluer, and without a proper ph profile the whole thing is just an unknown. I have quite aggressive growth of BBA and thread algae. I had to stop trimming off algae coated leaves on the buces, at the rate things were going I would have killed the plants. So I need to deal with the root cause before trimming off any more. I was never a fan of treating symptoms anyway, partly from principle and partly laziness. Ive been pretty good at manually removing hair algae recently, but it is competing with water changes for my very limited amount of energy, so im not sure if I am approaching that at the right end. I would think water changes are more important than manual removal of algae, but im not absolutely sure.
I did it! Figured out the dosing for the DIY remineralizer and got the spoon sizes and dosing instructions all written down for future Hufsa 
I bumped the final GH up from 6 to 7, I want to see if this makes the shrimp colony a bit more robust, especially with regard to water changes.
My brain didnt make any of the agonized creaking cogwheel noises it has been making lately, so I diagnose myself free of virus-induced smooth-brain. Now im back to normal stupid! 😃
I think I will be able to tackle micros tomorrow without setting myself on fire or killing everything I love. Using IFC calculator instead of rotala butterfly will leave a lot less room for errors as well. I just need to pick up some suitable storage bottles for the trace dilutions.
I dont want to have nickel solution sitting around in a soda bottle in the fridge, it might look too much like some kind of magical blue soda and my husband will definitely drink it and then die.
I will have to train a whole new one and thats just a lot of hassle
I bumped the final GH up from 6 to 7, I want to see if this makes the shrimp colony a bit more robust, especially with regard to water changes.
My brain didnt make any of the agonized creaking cogwheel noises it has been making lately, so I diagnose myself free of virus-induced smooth-brain. Now im back to normal stupid! 😃
I think I will be able to tackle micros tomorrow without setting myself on fire or killing everything I love. Using IFC calculator instead of rotala butterfly will leave a lot less room for errors as well. I just need to pick up some suitable storage bottles for the trace dilutions.
I dont want to have nickel solution sitting around in a soda bottle in the fridge, it might look too much like some kind of magical blue soda and my husband will definitely drink it and then die.
I will have to train a whole new one and thats just a lot of hassle
plantnoobdude
Member
I was in the same situation. was adding 1ppm plus of my edta micro mix, still had horrible Fe deficiency. swapped to one of happis recipe, 0.1ppm Fe dtpa per week and now plants are looking much much better. still have some issues, but not Fe deficiency! manganese deficiency presents it's self the same as Fe deficiency. Happi usually recommend Fe:Mn of 2:1. If you are using a premade mix for aquarium then you probably have too little Mn in relation to Fe.Gotta fix whatever this chlorosis thing is. Whats bugging me is that the sheer amount of iron that is currently being added should be more than sufficient. Its being added daily so theoretically I should be able to use whatever chelate I want. To just keep blindly adding more of the same when its obviously not working quite right seems like a mistake somehow. I should google how manganese deficiency presents
you can add some preservatives, ascorbic acid and pottasium sorbate. then you can store at room temp in a dark place. I would occasionally check to make sure there is no precipitation, and also draw lines on the edge of the bottle so that you know the stock solution isn't evaporating.I dont want to have nickel solution sitting around in a soda bottle in the fridge,
wait untill you see what the copper solution looks like!it might look too much like some kind of magical blue soda
I would also recommend double checking ALL numbers you get from the calculator. not only will it prevent some unknown mistakes, it will also help you practice the calculations for maths. not trying to doubt the calculator. but ofcourse it is not perfect.I think I will be able to tackle micros tomorrow without setting myself on fire or killing everything I love. Using IFC calculator
anyway, I am trying a new mix based on tropica numbers.
hopefully it works well.
Last edited:
Ive hit a little bit of a snag, I was mixing up trace solutions last night, and all went well except Boric Acid, which seems to take some time to dissolve. I thought leaving it over night would fix it but theres still some undissolved salt in the bottle this morning. Ive sat it in a hot bath right now to see if slightly higher temperature will make it happier. Does anyone know if this salt is particularly slow to dissolve, or have experiences with it? @dw1305 @Wookii or anyone else?
Details: I am attempting to dissolve 7,76 grams of Boric Acid into 500ml of demineralised water. This should be well within the solubility of Boric Acid.

I added the standard dose of ascorbic acid and potassium sorbate before adding the trace salt.
I tried checking if the preservatives could have maxed out the "available space" in the water, but can only see solubility for potassium sorbate at 100 degrees, which is a fair bit warmer than my kitchen.
Potassium sorbate: "Solubility in water 58.5 g/100 mL (100 °C)"
Also ascorbic acid only says "Solubility in water 330 g/L" with no mention of temperature, so I dont really know what to make of that.
Any ideas?
Details: I am attempting to dissolve 7,76 grams of Boric Acid into 500ml of demineralised water. This should be well within the solubility of Boric Acid.

I added the standard dose of ascorbic acid and potassium sorbate before adding the trace salt.
I tried checking if the preservatives could have maxed out the "available space" in the water, but can only see solubility for potassium sorbate at 100 degrees, which is a fair bit warmer than my kitchen.
Potassium sorbate: "Solubility in water 58.5 g/100 mL (100 °C)"
Also ascorbic acid only says "Solubility in water 330 g/L" with no mention of temperature, so I dont really know what to make of that.
Any ideas?
Attachments
Might have got it now. So ten minutes of patience would have worked instead of pinging everyone 😅 Sorry, my bad
Hot water bath seems to have done the trick, I guess maybe with the preservatives it was hovering right around max solubility, and our kitchen is not entirely room temperature, closer to 18 degrees if I had to guess
Hot water bath seems to have done the trick, I guess maybe with the preservatives it was hovering right around max solubility, and our kitchen is not entirely room temperature, closer to 18 degrees if I had to guess
Alright, so new fully DIY micro mix is set up and running, tank will get the first dose tomorrow morning with lights on (unless divine intervention)

Proud member of the "Forbidden Soda Club"
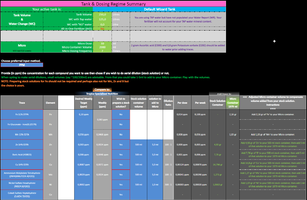
I decided to clone Tropica's trace ratio but just bumped it slightly up from recommended dosing, so instead of 0.08 Fe the entire thing is scaled up to 0.1 Fe. Tropica doesnt contain Ni (as far as I know?) so I pulled a little bit of an average from the various ratios I had in my notes and went for 0.0002 ppm.
Micro mix weekly (roughly Tropica x 1.2)
Fe 0.1 ppm (Fe 11% DTPA)
Mn 0.056 ppm (Mn 13% EDTA)
Zn 0.003 ppm (Zn 14% EDTA)
B 0.006 ppm (H3BO3)
Cu 0.0087 ppm (Cu 14% EDTA)
Mo 0.0029 ppm ((NH4)6Mo7O24.4(H2O))
Ni 0.0002 ppm (NiSO4.6(H2O))
@plantnoobdude and anyone else who might feel called, what color did your Mo solution turn out? Also did you use Ammonium molybdate as well? What about preservatives, did you add any? My Mo solution turned an interesting fresh lime green color when I first added it to the water with vit-c and p.sorbate, but then over night it turned the interesting dark jeans blue as pictured above. I am thinking it has reacted to the vit-c or the acidity somehow but hopefully not in a harmful way.
Performing a water change after lights off today, thinking of starting to front load parts of the weekly macros to keep things at a steady level. Will need to adjust autodoser amount to suit. Plan is to give the new micro mix around two weeks to show any changes, and then after that start gradually weaning the tank over to partly Urea for N. I didnt want to do everything quite at once.

Proud member of the "Forbidden Soda Club"
I decided to clone Tropica's trace ratio but just bumped it slightly up from recommended dosing, so instead of 0.08 Fe the entire thing is scaled up to 0.1 Fe. Tropica doesnt contain Ni (as far as I know?) so I pulled a little bit of an average from the various ratios I had in my notes and went for 0.0002 ppm.
Micro mix weekly (roughly Tropica x 1.2)
Fe 0.1 ppm (Fe 11% DTPA)
Mn 0.056 ppm (Mn 13% EDTA)
Zn 0.003 ppm (Zn 14% EDTA)
B 0.006 ppm (H3BO3)
Cu 0.0087 ppm (Cu 14% EDTA)
Mo 0.0029 ppm ((NH4)6Mo7O24.4(H2O))
Ni 0.0002 ppm (NiSO4.6(H2O))
@plantnoobdude and anyone else who might feel called, what color did your Mo solution turn out? Also did you use Ammonium molybdate as well? What about preservatives, did you add any? My Mo solution turned an interesting fresh lime green color when I first added it to the water with vit-c and p.sorbate, but then over night it turned the interesting dark jeans blue as pictured above. I am thinking it has reacted to the vit-c or the acidity somehow but hopefully not in a harmful way.
Performing a water change after lights off today, thinking of starting to front load parts of the weekly macros to keep things at a steady level. Will need to adjust autodoser amount to suit. Plan is to give the new micro mix around two weeks to show any changes, and then after that start gradually weaning the tank over to partly Urea for N. I didnt want to do everything quite at once.