Another noob question.
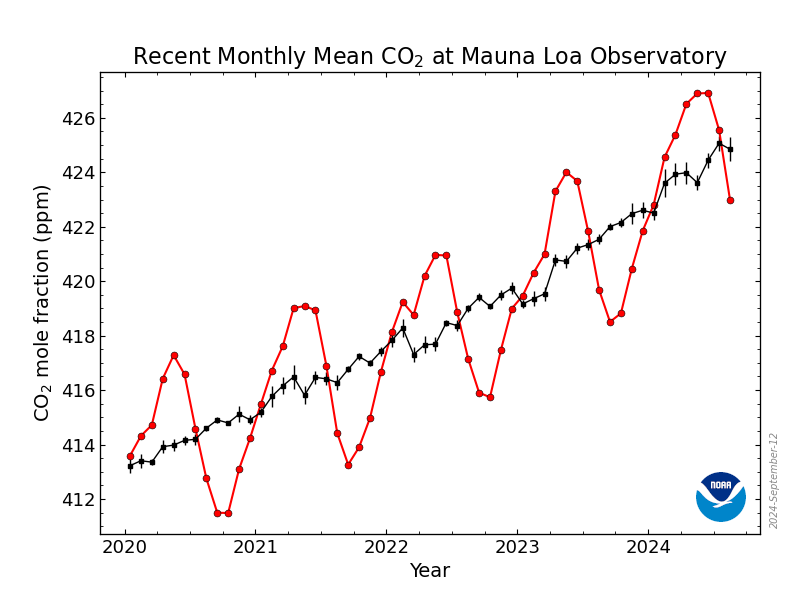
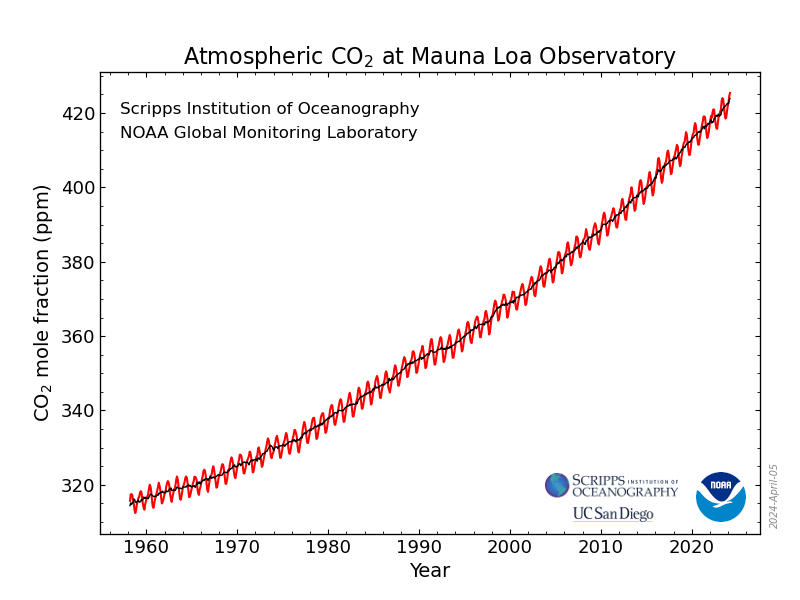
Hypothetically: there's a lots of CO2 in the air, about 400 ppm.
When you dose this air into a tank by, an air pump and a diffuser, then you automatically get higher CO2 values, right?
So why we add CO2 in gaseous form. Is that more concentrated or something? 🤔
Hypothetically: there's a lots of CO2 in the air, about 400 ppm.
When you dose this air into a tank by, an air pump and a diffuser, then you automatically get higher CO2 values, right?
So why we add CO2 in gaseous form. Is that more concentrated or something? 🤔