dino21
Member
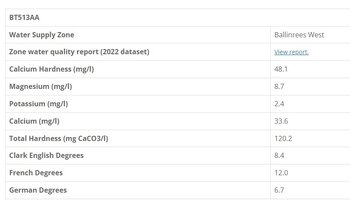
Think we would get a simple GH /KH test kit and establish whats in the water coming through your tap; then dependent on what folk suggest is a good hardness level for the stock you intend to keep, will determine the best way to prepare your water.
Not a product we have used but this good supplier stock this brand -
JBL Aquadur
Not a product we have used but this good supplier stock this brand -
JBL Aquadur
Last edited: