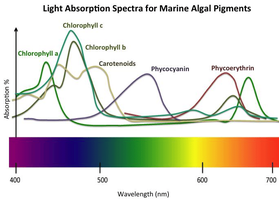
I mean, there is a graph of light absorption by plants and each spectrum has its maximum: for example, chlorophyll c has it in the region of 85% about 480nm. This means that if 200 umol/m2*s 480nm falls on it, the plant will absorb 170 umol/m2*s, well, but if 400 umol/m2*s falls, 340 umol/m2*s will be absorbed. That is, the question here is that in any case will any amount of energy be released? Why does it work like this? Or do I not understand something? Thanks!
-
You are viewing the forum as a Guest, please login (you can use your Facebook, Twitter, Google or Microsoft account to login) or register using this link: Log in or Sign Up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What prevents plants from absorbing all spectrum?
- Thread starter MiZubov
- Start date

5.2 The Light-Dependent Reactions of Photosynthesis - Concepts of Biology | OpenStax
The sun emits an enormous amount of electromagnetic radiation (solar energy). Humans can see only a fraction of this energy, which is referred to as “vi...
Photosynthetic efficiency - Wikipedia
In actuality, however, plants do not absorb all incoming sunlight (due to reflection, respiration requirements of photosynthesis and the need for optimal solar radiation levels) and do not convert all harvested energy into biomass, which results in a maximum overall photosynthetic efficiency of 3 to 6% of total solar radiation.[1] If photosynthesis is inefficient, excess light energy must be dissipated to avoid damaging the photosynthetic apparatus. Energy can be dissipated as heat (non-photochemical quenching), or emitted as chlorophyll fluorescence.
Andy Pierce
Member
I'm not sure what the question is... light falls on plant and either the light is reflected away, or the light is absorbed. If the light is absorbed there are three possibilities:
Short version: light falls on the plant and that fraction absorbed is converted into heat and various chemical components of the plant.
The chlorophyll fluorescence is small - 1-2% of light absorbed. The rest of the light energy either goes into making/breaking chemical bonds e.g. CO2 + water -> glucose + O2 or into heat directly. The glucose is either 'chemically burned' which releases energy (more heat), and/or used to synthesize structural molecules (starch, cellulose) or if you provide a nitrogen source, proteins. (Photosynthesis - Equation, Formula & Products | ChemTalk).Light energy absorbed by chlorophyll molecules in a leaf can undergo one of three fates: it can be used to drive photosynthesis (photochemistry), excess energy can be dissipated as heat or it can be re‐emitted as light—chlorophyll fluorescence. (Chlorophyll fluorescence—a practical guide)
Short version: light falls on the plant and that fraction absorbed is converted into heat and various chemical components of the plant.
In actuality, however, plants do not absorb all incoming sunlight (due to reflection, respiration requirements of photosynthesis and the need for optimal solar radiation levels) and do not convert all harvested energy into biomass, which results in a maximum overall photosynthetic efficiency of 3 to 6% of total solar radiation.
This article says that only a small part of the sunlight is captured by plants, and the rest is either reflected or passes into heat and the like. The sorption of sunlight is depicted on my graph, but my question is whether these graphs change when plants are illuminated by an artificial light source, not the sun.
Here is an article that shows how different wavelengths affect plants and that it happens differently depending on their combination.
But my most important question is whether the peak values of different pigments change depending on the lighting power. Why should this happen? The sun is a very powerful source of illumination, so some of the energy is "spat out" by plants, but our aquarium lamps are much weaker, it is possible that the peaks, for example, of carotenoids become much higher due to weak lighting (or, conversely, they are poorly activated due to weak light)?
Hi all,
<"How the Optical Properties of Leaves Modify the Absorption and Scattering of Energy and Enhance Leaf Functionality">*
*Ustin, S.L., Jacquemoud, S. (2020). How the Optical Properties of Leaves Modify the Absorption and Scattering of Energy and Enhance Leaf Functionality. In: Cavender-Bares, J., Gamon, J.A., Townsend, P.A. (eds) Remote Sensing of Plant Biodiversity. Springer, Cham.
cheers Darrel
I think that is likely, you are also going to get internal reflection etc., with light scattering and changes in wavelength etcThe sun is a very powerful source of illumination, so some of the energy is "spat out" by plants, but our aquarium lamps are much weaker, it is possible that the peaks, for example, of carotenoids become much higher due to weak lighting (or, conversely, they are poorly activated due to weak light)?
<"How the Optical Properties of Leaves Modify the Absorption and Scattering of Energy and Enhance Leaf Functionality">*
*Ustin, S.L., Jacquemoud, S. (2020). How the Optical Properties of Leaves Modify the Absorption and Scattering of Energy and Enhance Leaf Functionality. In: Cavender-Bares, J., Gamon, J.A., Townsend, P.A. (eds) Remote Sensing of Plant Biodiversity. Springer, Cham.
cheers Darrel
Andy Pierce
Member
No. The graph is a measure of absorption of the isolated chemical chlorophylls in solvent with respect to wavelength (the usual method for absorption spectra). It doesn't have anything to do with the source of the light, or with leaves either. See also: Chlorophyll pigments are bluer than we thinkmy question is whether these graphs change when plants are illuminated by an artificial light source, not the sun
This means that whatever the lighting power, chlorophyll b at 480nm will absorb 80% of the fallen light, regardless of the power of the lighting source?No. The graph is a measure of absorption of the isolated chemical chlorophylls in solvent with respect to wavelength (the usual method for absorption spectra). It doesn't have anything to do with the source of the light, or with leaves either. See also: Chlorophyll pigments are bluer than we think
Could you give me a link to this resource?the usual method for absorption spectra
No, you can saturate the system.This means that whatever the lighting power, chlorophyll b at 480nm will absorb 80% of the fallen light, regardless of the power of the lighting source?
Photoinhibition occurs when light exceeds physiological saturation and results in an excess of photons that are not dissipated by photosynthetic carbon fixation or by fluorescence or quenching reactions (Long et al.
There are organisms that we haven't found the saturation point yet.
Cannabis and Giant clams for example.
Keep in mind the absorption curves of various pigments ( chl, carotenoids, xanthophylls ) overlap.
Hi all,
However <"you can fry"> Anubias barteri and Bolbitis heudelotii etc. with <"plenty of light">.
<"Really dark green"> plants have plenty of chlorophyll (a and b) and once they've intercepted the light that energy has to "go" somewhere.
cheers Darrel
That makes sense. The thing you have to remember with photosynthesis is that it <"only evolved once"> and all photosynthetic organisms share a <"common ancestor (and chlorophyll a)">.The graph is a measure of absorption of the isolated chemical chlorophylls
I didn't know that either, but it gives us some more <"turned up to eleven"> plants (the <"Clams Zooxanthellae">).There are organisms that we haven't found the saturation point yet.
Cannabis and Giant clams for example.
However <"you can fry"> Anubias barteri and Bolbitis heudelotii etc. with <"plenty of light">.
<"Really dark green"> plants have plenty of chlorophyll (a and b) and once they've intercepted the light that energy has to "go" somewhere.
cheers Darrel
Last edited:
Andy Pierce
Member
2.1.5: Spectrophotometry is a good starting place. I suspect that in the graph you originally posted they have mislabelled the Y-axis as 'Absorption %' when it should be labelled 'Absorbance'. The difference in meaning between these two terms is left as an exercise for the reader...Could you give me a link to this resource
I have written that article. To the point.2.1.5: Spectrophotometry is a good starting place. I suspect that in the graph you originally posted they have mislabelled the Y-axis as 'Absorption %' when it should be labelled 'Absorbance'. The difference in meaning between these two terms is left as an exercise for the reader...
Conclusions: that graph shows absorbance of different substances on the Y-axis - you were really right!
But, I highlighted one interesting thing from that article, these graphs only show that green things reflect green wavelengths. But it does not follow in any way that plants do not need green light (although this graph us want showed exactly that).
Green plants use green light for photosynthesis.I have written that article. To the point.
Conclusions: that graph shows absorbance of different substances on the Y-axis - you were really right!
But, I highlighted one interesting thing from that article, these graphs only show that green things reflect green wavelengths. But it does not follow in any way that plants do not need green light (although this graph us want showed exactly that).
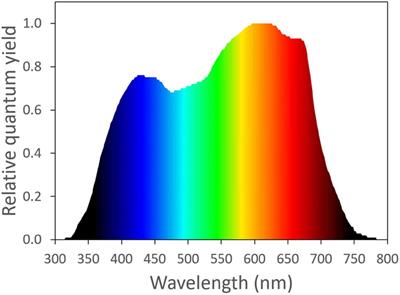
Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms
Red and blue light are traditionally believed to have a higher quantum yield of CO2 assimilation (QY, moles of CO2 assimilated per mole of photons) than green light, because green light is absorbed less efficiently. However, because of its lower absorptance, green light can penetrate deeper and...
Hello!
I want to clarify about the PPP and PPPD parameters.
PPF — photosynthetic photons flux — the number of photons directly affecting chlorophyll a, b and carotenoids. This is a characteristic of the lamp, without taking the area to which the lighting extends. It is measured in micromole /s. That is, the amount of matter (in this case photons) per unit of time that is emitted by the illumination source.
PPFD — photosynthetic photons flux density — the number of photons in the range of 400-700 nm received by the surface over a certain period of time, or the flux density of photosynthetic photons (PPFD) in units /m2*s
How to operate correctly with the PPF parameter, which is marked on the lamp?
Let's say 300 is specified.
I take my aquarium area, say, 0.5 m2 and divide 300 by 0.5 m2 and get 600.
Does this mean 600 umol/m2*s I get directly under the lamp at one point? I'd like to understand, what the role plays m2.
Thanks a lot!
I want to clarify about the PPP and PPPD parameters.
PPF — photosynthetic photons flux — the number of photons directly affecting chlorophyll a, b and carotenoids. This is a characteristic of the lamp, without taking the area to which the lighting extends. It is measured in micromole /s. That is, the amount of matter (in this case photons) per unit of time that is emitted by the illumination source.
PPFD — photosynthetic photons flux density — the number of photons in the range of 400-700 nm received by the surface over a certain period of time, or the flux density of photosynthetic photons (PPFD) in units /m2*s
How to operate correctly with the PPF parameter, which is marked on the lamp?
Let's say 300 is specified.
I take my aquarium area, say, 0.5 m2 and divide 300 by 0.5 m2 and get 600.
Does this mean 600 umol/m2*s I get directly under the lamp at one point? I'd like to understand, what the role plays m2.
Thanks a lot!
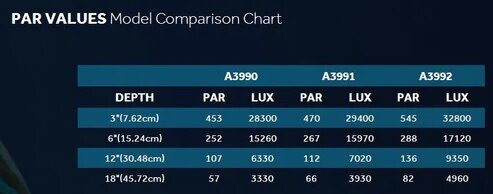
Because there isn't.And one more question is.
Why do we need all these unnecessary difficulties if there is a direct relationship between PAR and LUX (Judging by this table)?
Play with this calculator.
Convert Lux to PPFD - Online Calculator | Waveform Lighting
Online calculator to convert illuminance (lux) to PPFD (micromoles per second per meter squared).