-
You are viewing the forum as a Guest, please login (you can use your Facebook, Twitter, Google or Microsoft account to login) or register using this link: Log in or Sign Up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
RO analysis
- Thread starter egor66
- Start date
Hi all,
Welcome to UKAPS,
Conductivity meters are quite useful <"Invert suggestions"> for an over-view of the water.
Reverse Osmosis doesn't include any <"ion exchange"> (so you haven't swapped a calcium (Ca++) for two sodium ions (Na+)), a really good RO unit should remove all ions, and your unit has done a pretty good job.
If you wanted even purer water? You would need a DI unit (RO plus an ion exchange filter), but I don't think you need it (in fact I don't think you need the RO unit).
cheers Darrel
Welcome to UKAPS,
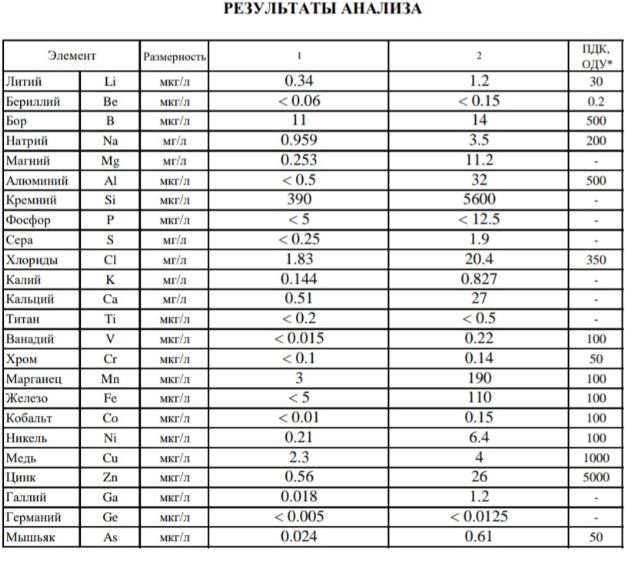
To be honest it is really pure water straight out of the tap. If you look at the units you have "milligrams / litre" (ppm or 10^-6) and "micrograms / litre" (ppb or 10^-9), so they are all really low values from the tap.
Conductivity meters are quite useful <"Invert suggestions"> for an over-view of the water.
Reverse Osmosis doesn't include any <"ion exchange"> (so you haven't swapped a calcium (Ca++) for two sodium ions (Na+)), a really good RO unit should remove all ions, and your unit has done a pretty good job.
If you wanted even purer water? You would need a DI unit (RO plus an ion exchange filter), but I don't think you need it (in fact I don't think you need the RO unit).
cheers Darrel
Thank you for your reply.in fact I don't think you need the RO unit
The thing is that the carbonate hardness of tap water is 4 units. I am trying to keep plants of the tonina genus.
So I have to purify the water, then add hardness salts up to gh5. Accordingly, kh remains 0-1.
However, I cannot grow plants. I have problems stopping growth points. Leaf curling.
This is provided that there is good light, intensive supply of carbon dioxide and application of fertilizers according to the estimative index system.
So I am thinking. Perhaps low NA values of 0.1ppm are bad and should I add a little?
Attachments
Hi all,
You can always deplete (just) the alkalinity (dKH - carbonate hardness) with an acid addition. @Andy Pierce uses 1 mol. hydrochloric acid (HCl) <"Tropica soil raising my KH">, but he understands what he is doing and <"I prefer citric acid (C6H8O7)"> mainly because I'm a pretty poor scientist. I'd also had some humic compounds (from dead Oak (Quercus) leaves or Alder (Alnus) "cones" <"All the leaves are brown… — Seriously Fish">).
I think that you may have iron (Fe) deficiency, have a look at: <"What is the “Duckweed Index” all about?">
cheers Darrel
That would make sense - <"KH <= GH">.The thing is that the carbonate hardness of tap water is 4 units. I am trying to keep plants of the tonina genus.
You can always deplete (just) the alkalinity (dKH - carbonate hardness) with an acid addition. @Andy Pierce uses 1 mol. hydrochloric acid (HCl) <"Tropica soil raising my KH">, but he understands what he is doing and <"I prefer citric acid (C6H8O7)"> mainly because I'm a pretty poor scientist. I'd also had some humic compounds (from dead Oak (Quercus) leaves or Alder (Alnus) "cones" <"All the leaves are brown… — Seriously Fish">).
I actually think that is your problem, you need a lot fewer calcium (Ca++) and magnesium (Mg++) ions and less hardness (dGH) all around. You are going to add magnesium (Mg) with your fertiliser addition and you only need to add a very minimal amount of calcium (Ca) (via CaSO4.2H2O or CaCl.2H2O).So I have to purify the water, then add hardness salts up to gh5.
I think that you may have iron (Fe) deficiency, have a look at: <"What is the “Duckweed Index” all about?">
Estimative Index? I'd use much, much leaner dosing, these are plants from very nutrient (and base) poor environments.This is provided that there is good light, intensive supply of carbon dioxide and application of fertilizers according to the evaluation index system.
Plants don't have an absolute sodium (Na) requirement, so I don't think it is a problem.Perhaps low NA values of 0.1ppm are bad and should I add a little?
cheers Darrel
Last edited:
Hi all,
That would make sense - <"KH <= GH">.
You can always deplete (just) the alkalinity (dKH - carbonate hardness) with an acid addition. @Andy Pierce uses 1 mol. hydrochloric acid (HCl) <"Tropica soil raising my KH">, but he understands what he is doing and <"I prefer citric acid (C6H8O7)"> mainly because I'm a pretty poor scientist. I'd also had some humic compounds (from dead Oak (Quercus) leaves or Alder (Alnus) "cones" <"All the leaves are brown… — Seriously Fish">).
I actually think that is your problem, you need a lot fewer calcium (Ca++) and magnesium (Mg++) ions and less hardness (dGH) all around. You are going to add magnesium (Mg) with your fertiliser addition and you only need to add a very minimal amount of calcium (Ca) (via CaSO4.2H2O or CaCl.2H2O).
I think that you may have iron (Fe) deficiency, have a look at: <"What is the “Duckweed Index” all about?">
Estimative Index? I'd use much, much leaner dosing, these are plants from very nutrient (and base) poor environments.
Plants don't have an absolute sodium (Na) requirement, so I don't think it is a problem.
cheers Darrel
Forgot to clarify. I use soil ista.Estimative Index?
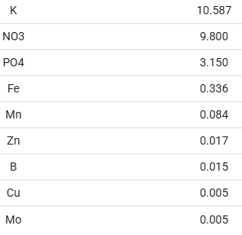
Regarding gh. More precisely Ca 24 ppm, Mg 7 ppm.
I apply slightly less fertilizer than recommended.
weekly dosage.
Attachments
Hi all,
cheers Darrel
Do you have these as separate salts? (MgSO4.7H2O etc) or are they via a commercial mix? If they are separate salts? Just add ~ 5 ppm (mg / l) of both minerals.Regarding gh. More precisely Ca 24 ppm, Mg 7 ppm.
I had to look that one up, do you mean the liquid fertilizer? <"Soil & Fertilizer_Water Plant System_Water Plant Fertilizer_Products | ISTA Taiwan _ Tzong-Yang Aquarium"> or <"Soil & Fertilizer_Water Plant System_Premium Water Plant Fertilizer_Products | ISTA Taiwan _ Tzong-Yang Aquarium">?I use soil ista.
cheers Darrel
Absolutely right. They use dry salts. Calcium sulfate and magnesium sulfate.Hi all,
Do you have these as separate salts? (MgSO4.7H2O etc) or are they via a commercial mix? If they are separate salts? Just add ~ 5 ppm (mg / l) of both minerals.
I had to look that one up, do you mean the liquid fertilizer? <"Soil & Fertilizer_Water Plant System_Water Plant Fertilizer_Products | ISTA Taiwan _ Tzong-Yang Aquarium"> or <"Soil & Fertilizer_Water Plant System_Premium Water Plant Fertilizer_Products | ISTA Taiwan _ Tzong-Yang Aquarium">?
cheers Darrel
Substrate ista