lisabeeren
New Member
hi,
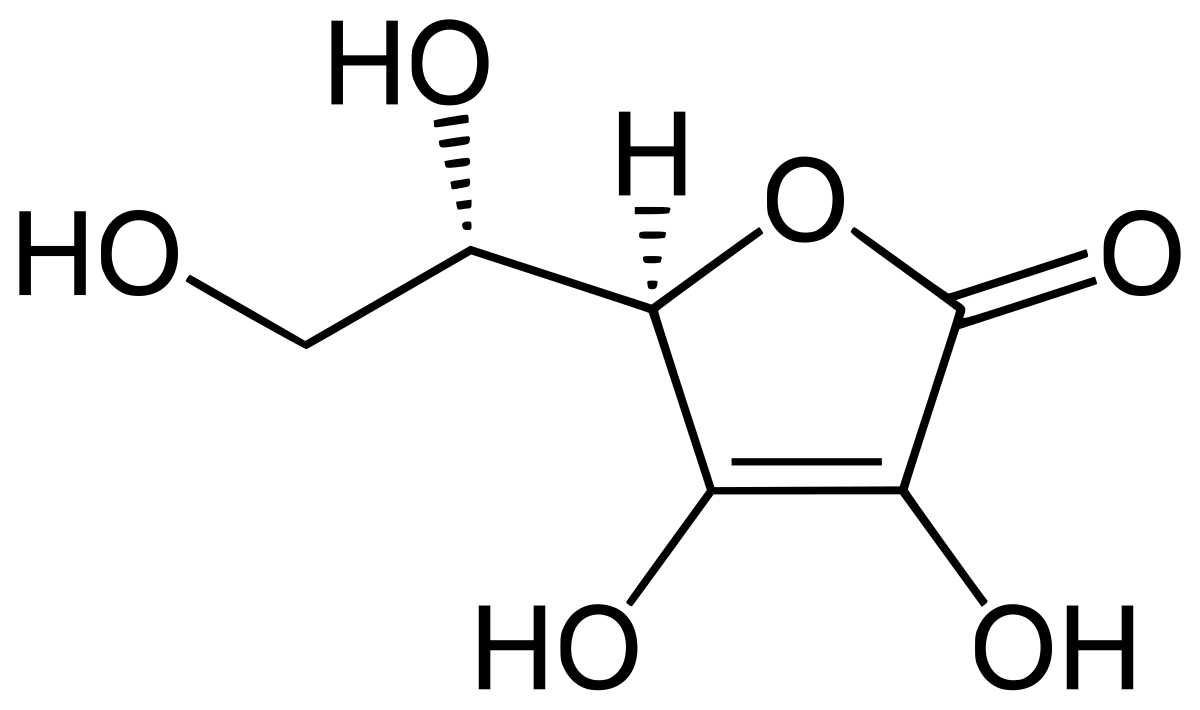
i've mixed up some solutions of macros, which i then combine in different proportions to achieve the NPK ratio i'm after. i start with deionised water, add 500mg ascorbic per L, 400mg potassium sorbate per L, wait until that completely dissolves, before adding MKP (monopotassium phosphate).
this all works, but after a couple of weeks, the solution has gradually changed colour (see attached image).
any idea what reaction might be taking place here?
i have found a few threads where people are complaining about their macros changing colour, but no definitive conclusion. i suppose my contribution here might be narrowing it down to MKP.
with thanks
i've mixed up some solutions of macros, which i then combine in different proportions to achieve the NPK ratio i'm after. i start with deionised water, add 500mg ascorbic per L, 400mg potassium sorbate per L, wait until that completely dissolves, before adding MKP (monopotassium phosphate).
this all works, but after a couple of weeks, the solution has gradually changed colour (see attached image).
any idea what reaction might be taking place here?
i have found a few threads where people are complaining about their macros changing colour, but no definitive conclusion. i suppose my contribution here might be narrowing it down to MKP.
with thanks