Morning,
I’ve just done some testing on my tank water and I’m not sure if I need to address matters.
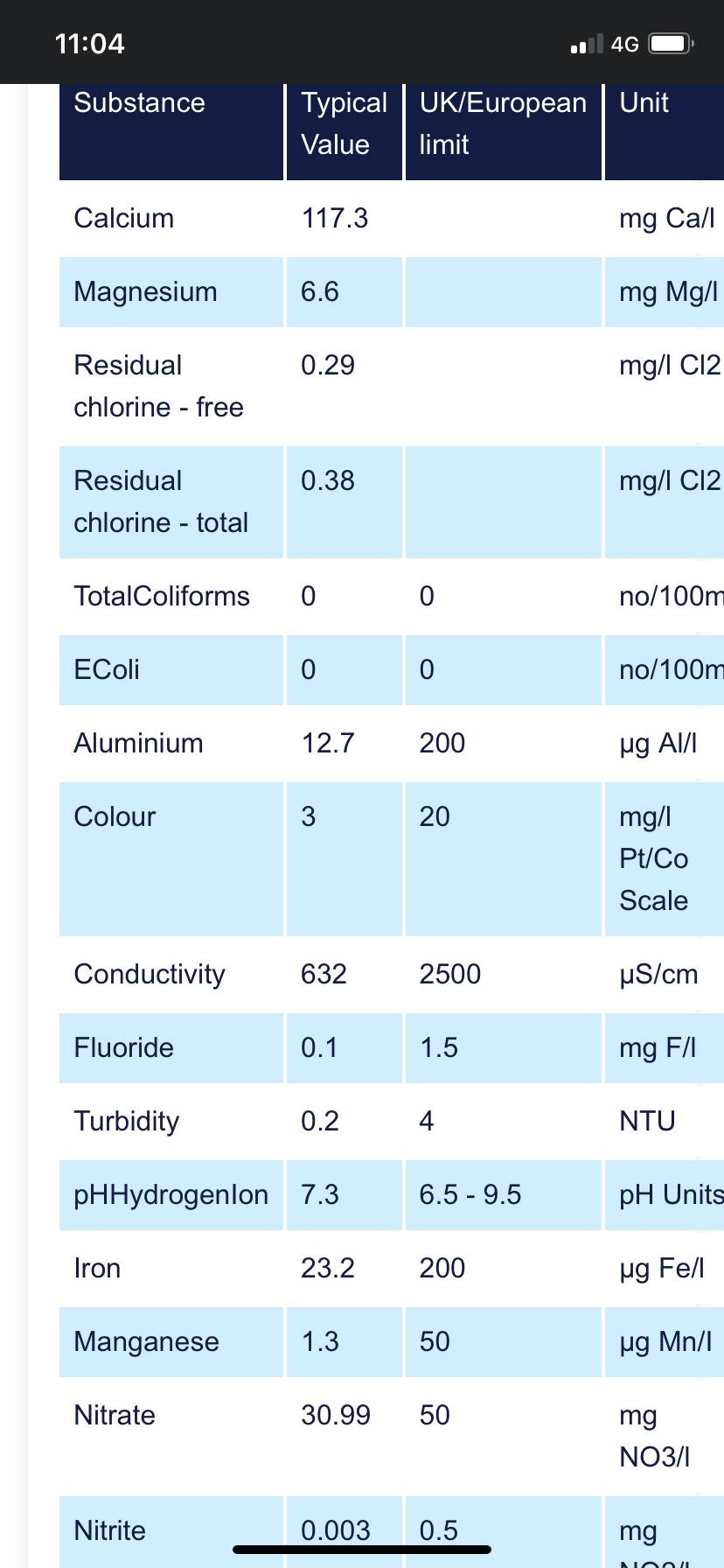
I have a journal thread on this website but thought I’d post this query here. So, my tanks pH is coming up paler than the colour for 6.4 (so pH is lower than this) and KH is the same colour as 0d (both are Tetra test kits).
My tap water has pH around 6.8 and KH of 6d.
I use Seachem Prime for water changes. I don’t inject co2.
The tank is almost 4 weeks old and with several ‘easy’ plants.
Are these drops in numbers a concern that need to be remedied?
Many thanks.
I’ve just done some testing on my tank water and I’m not sure if I need to address matters.
I have a journal thread on this website but thought I’d post this query here. So, my tanks pH is coming up paler than the colour for 6.4 (so pH is lower than this) and KH is the same colour as 0d (both are Tetra test kits).
My tap water has pH around 6.8 and KH of 6d.
I use Seachem Prime for water changes. I don’t inject co2.
The tank is almost 4 weeks old and with several ‘easy’ plants.
Are these drops in numbers a concern that need to be remedied?
Many thanks.