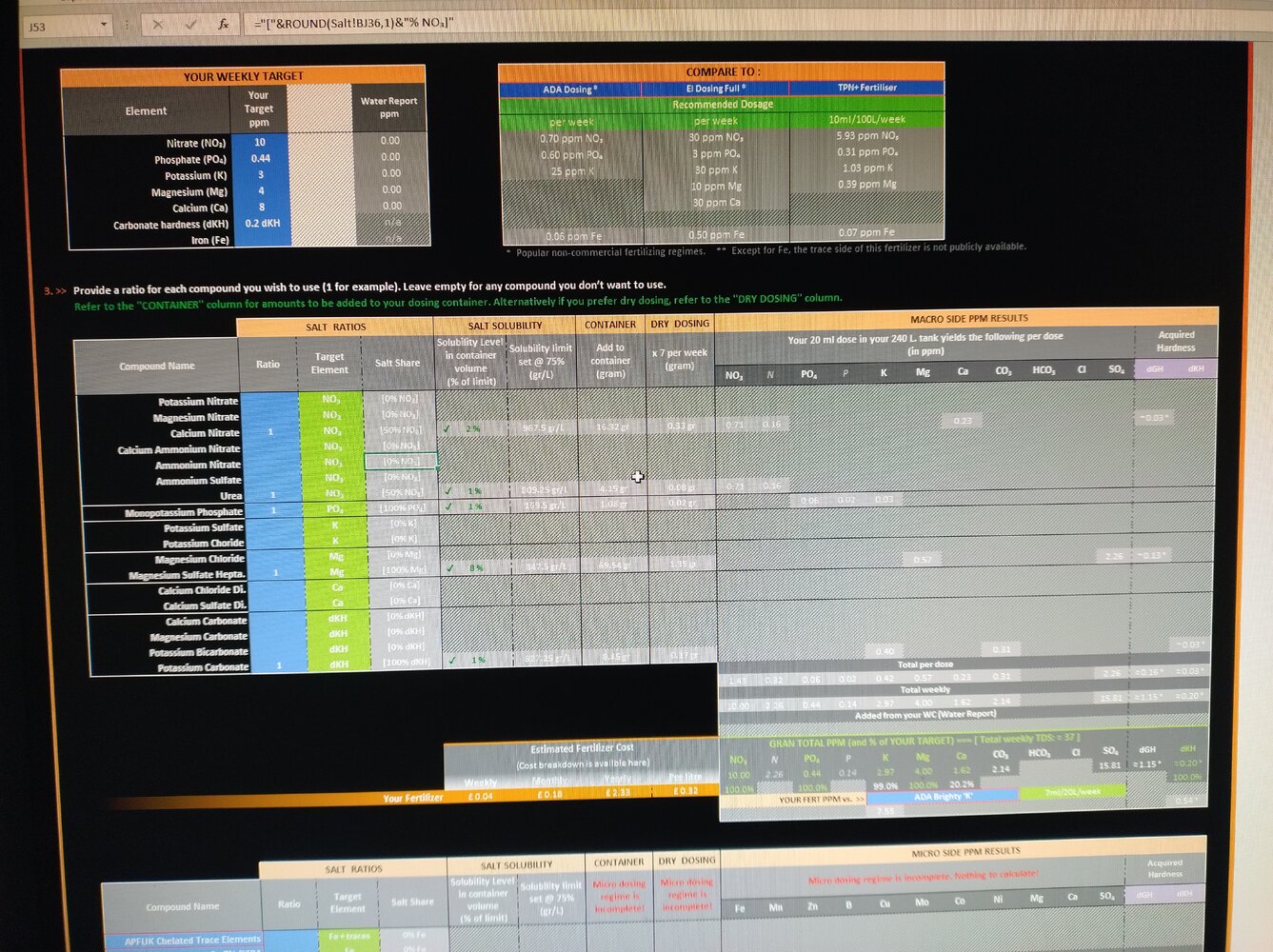
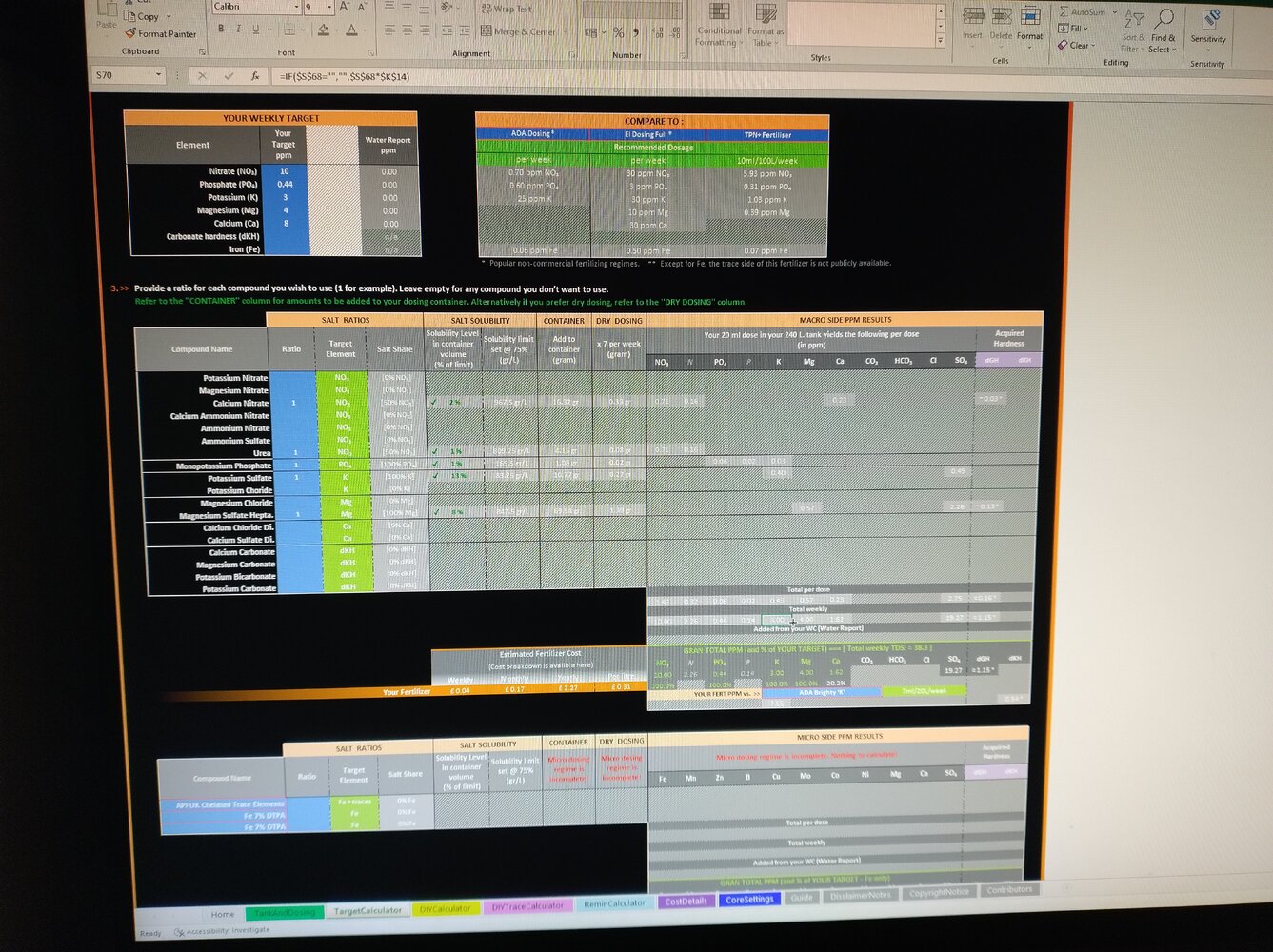
I am going away for a month and need to create a dosing solution for when I am away. I usually dose each compound separately twice per week, so no issues here. I bought a dosing pump and can dose micro/macro. The problem I have, when I use IFC calculator and add everything together solution turns into milk, so I guess my solution turns useless. I do not think I'm adding to much, trying to be sensible... Could someone please let me know what I am doing wrong? Below is the screenshot of what I want to achieve. Many thanks, Matt.
-
You are viewing the forum as a Guest, please login (you can use your Facebook, Twitter, Google or Microsoft account to login) or register using this link: Log in or Sign Up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Help with macro solution
- Thread starter palcente
- Start date
hypnogogia
Member
Doing it as an AIO is resulting in chemical reaction with something precipitating out of solution. Someone better at chemistry will be along and be able to tell you what.
Edit: At a guess, it’s the calcium nitrate that’s reacting with something else.
Edit: At a guess, it’s the calcium nitrate that’s reacting with something else.
John q
Member
I think your problem is trying to mix Calcium nitrate and Magnesium Sulfate together in the same bottle. Try emitting the Magnesium and see if that helps, you can add the Mg in with the micro solution.
Edit: just seen you're also using potassium sulfate too, so you need to remove the Calcium nitrate from the mix.
Edit: just seen you're also using potassium sulfate too, so you need to remove the Calcium nitrate from the mix.
MichaelJ
Member
Yes, both will precipitate.potassium sulfate too, so you need to remove the Calcium nitrate
Looping in @Hanuman ... not sure if it's feasible (or if it's already in there) but it would be cool to have the IFC Calculator warn you if you specify a mix of compounds that wont play well together.
Cheers,
Michael
Last edited:
@palcente
#1
calcium nitrate (highly react with CO3)
Urea
Monopotassium phosphate (can cause issues)
Magnesium sulphate (source of So4, also react with CO3)
potassium carbonate (source of CO3)
#2
calcium nitrate (react with SO4)
urea
Monopotassium phosphate (can cause issues)
potassium sulfate (source of So4)
Magnesium sulphate (source of So4)
anytime you add any source of Ca or Mg with CO3, lets say your solution contain KHCO3 or K2CO3 it will form precipitation, it will form CaCO3, MgCO3 and fall out of the solution. if you were to add any source of Ca or Mg to your solution that contain SO4, then it will form CaSO4 and fall out of the solution, the solubility of CaSO4 is also very very low, while Mg will dissolve.
its probably not the best practice to use P and Ca in the same solution. however, they can be in the same solution if the solution is kept very acidic. there are several chemicals that are not meant to be put together in the solution but sometime those rules can be broken and making the solution very acidic is one way to do it.
#1
calcium nitrate (highly react with CO3)
Urea
Monopotassium phosphate (can cause issues)
Magnesium sulphate (source of So4, also react with CO3)
potassium carbonate (source of CO3)
#2
calcium nitrate (react with SO4)
urea
Monopotassium phosphate (can cause issues)
potassium sulfate (source of So4)
Magnesium sulphate (source of So4)
anytime you add any source of Ca or Mg with CO3, lets say your solution contain KHCO3 or K2CO3 it will form precipitation, it will form CaCO3, MgCO3 and fall out of the solution. if you were to add any source of Ca or Mg to your solution that contain SO4, then it will form CaSO4 and fall out of the solution, the solubility of CaSO4 is also very very low, while Mg will dissolve.
its probably not the best practice to use P and Ca in the same solution. however, they can be in the same solution if the solution is kept very acidic. there are several chemicals that are not meant to be put together in the solution but sometime those rules can be broken and making the solution very acidic is one way to do it.
As Happy said, keep those compounds separate. Even if keeping them together under some specific condition is possible, I would advise against it. You will note that no liquid fertilizer manufacturer keep these compounds in their commercial macro mix.
My opinion is that since you are preparing this temporary mix due to you being away, I would probably just slightly overdose your regular Ca compound (probably CaCl2 or CaSO4) before leaving. I don't think it will have a negative impact on the system. Same for the carbonate. Alternatively you could just add some small piece of cuttlebone and be done with it. Then prepare your normal macro solution without these two compounds. Replace your Calcium Nitrate and Potatium Carbonate with only Potassium Nitrate. You will overshoot your K target but only by a very small margin. 3.3ppm vs 3ppm. Nothing to lose your sleep over and certainly nothing that will affect your plants and fauna. This will also keep the same ratio betwen nitrate and urea (50/50).
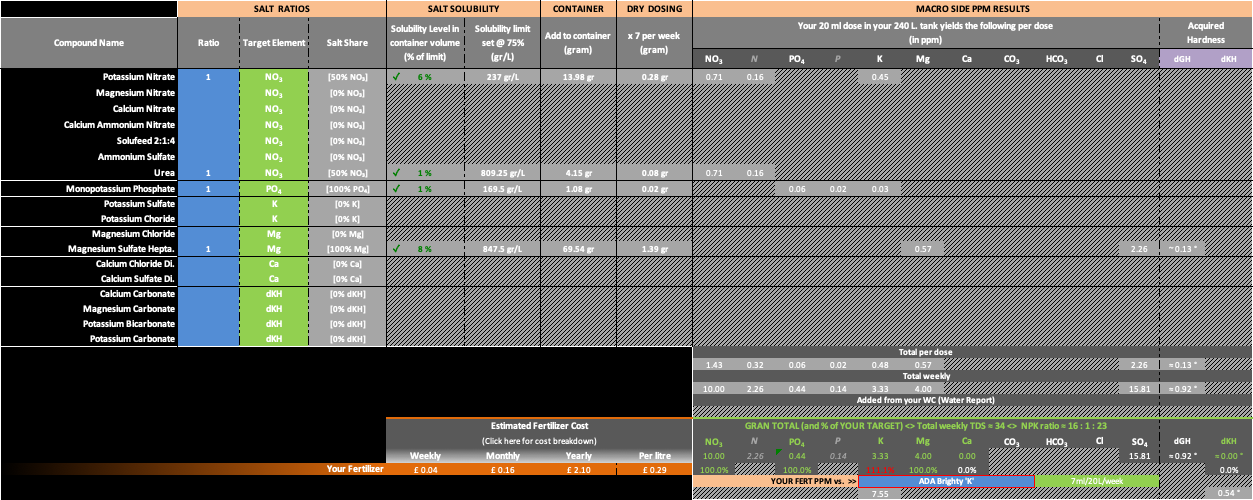
If you are adamant to keeping that K up to an exact 3ppm, then I did a Goal Seek search in Excel which resulted in the following Potassium Nitrate dosing. However this will have the effect of slightly increasing the Urea ratio
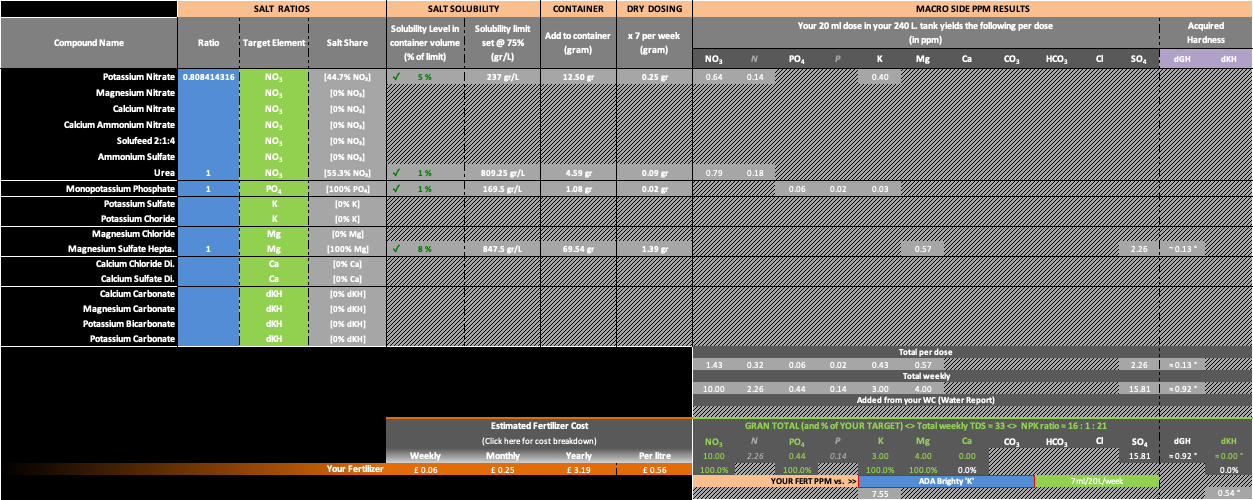
 .
.
My opinion is that since you are preparing this temporary mix due to you being away, I would probably just slightly overdose your regular Ca compound (probably CaCl2 or CaSO4) before leaving. I don't think it will have a negative impact on the system. Same for the carbonate. Alternatively you could just add some small piece of cuttlebone and be done with it. Then prepare your normal macro solution without these two compounds. Replace your Calcium Nitrate and Potatium Carbonate with only Potassium Nitrate. You will overshoot your K target but only by a very small margin. 3.3ppm vs 3ppm. Nothing to lose your sleep over and certainly nothing that will affect your plants and fauna. This will also keep the same ratio betwen nitrate and urea (50/50).
If you are adamant to keeping that K up to an exact 3ppm, then I did a Goal Seek search in Excel which resulted in the following Potassium Nitrate dosing. However this will have the effect of slightly increasing the Urea ratio
It could be done as we are already doing it for some other highly insolubable compounds such as Calcium Sulfate, Calcium Carbonate and Magnesium Carbonate. Problem lays on the fact that we are not chemists so it becomes tricky to know all the interactions between all compounds. And even if we did know then it becomes a labyrinth because all compound have the ability to react in high enough amounts. Monopotassium Phosphate for example is one. Also, thechnically this is a Calculator, not an AI assistant 😛 but we do provide some tips right and left when we thought it was necessary. Finally, this allows people to learn the hard way and it justifies us answering to this thread with detailed explanationsbut it would be cool to have the IFC Calculator warn you if you specify a mix of compounds that wont play well together.
Last edited: