Hi all,
What @LFNfan says,
You can think about it like money, nitrifying bacteria change ten individual pound coins into a £10 pound note, but it is still £10 and you need to add some more money, if that makes sense?
cheers Darrel
What @LFNfan says,
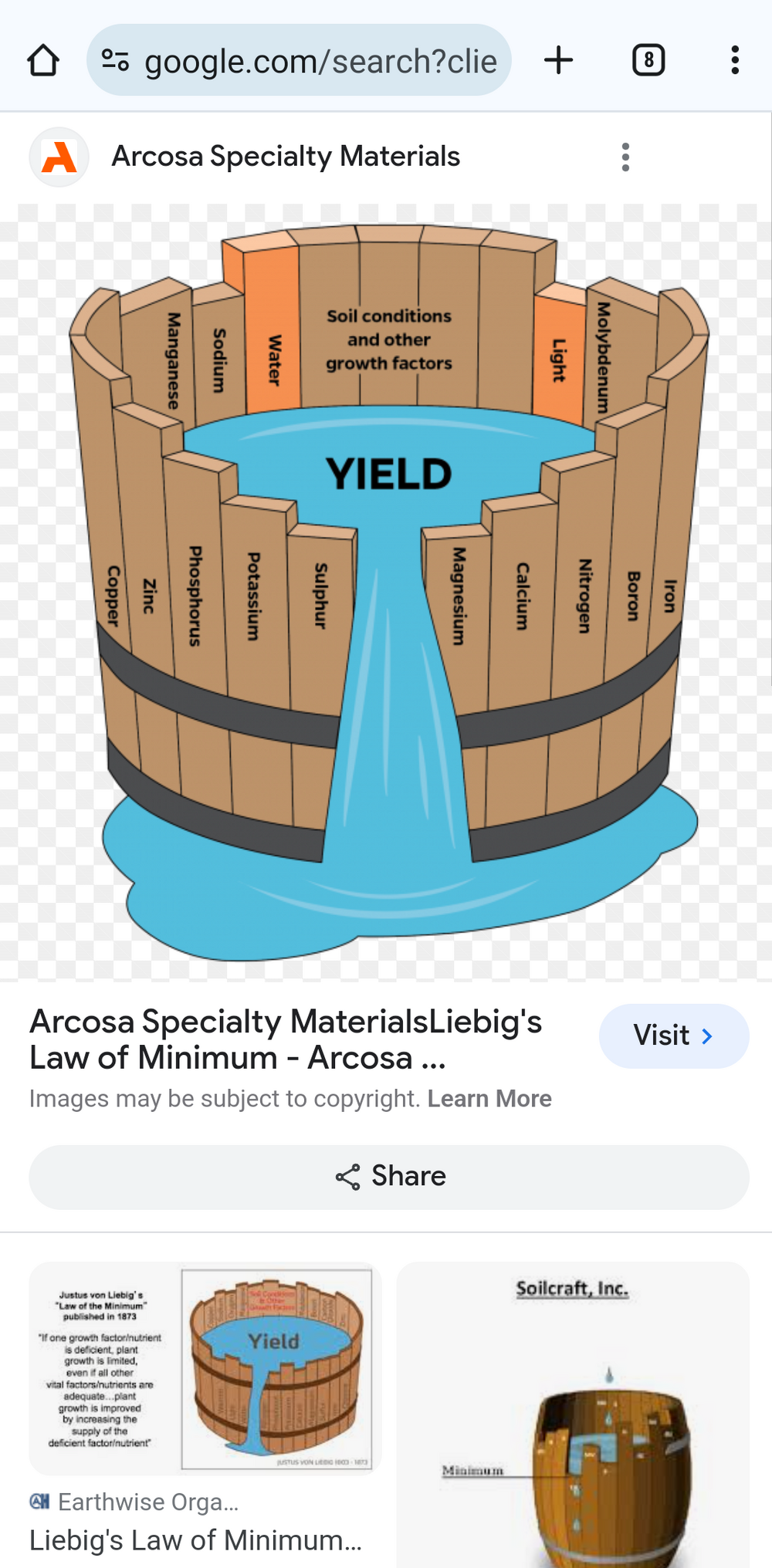
but I'll still post this.Flourish and beneficial bacteria are not going to provide what's needed to feed your plants properly (macro nutrients N, P, K and micro nutrients like Fe) and with your water so pure the plants are just about surviving on fumes (fish pee).
If they worked (and that is very much open to question <"Dr Timothy Hovanec's comments about Bacterial supplements">) they would provide ammonia oxidising bacteria (AOB) that convert TAN ammonia / ammonium (NH3 / NH4+) to nitrite (NO2-) and nitrite oxidising bacteria (NOB) that convert NO2- to nitrate (NO3-). They don't create any <"new fixed nitrogen">, they just change it from one form to another.and I’ve tried adding “beneficial bacteria” but it doesn’t seem to change my readings much!
You can think about it like money, nitrifying bacteria change ten individual pound coins into a £10 pound note, but it is still £10 and you need to add some more money, if that makes sense?
cheers Darrel
Last edited: