dallen98
Member
Hello friends, it's been a while. Shortly after getting my aquariums up and running in my previous journal, a rare opportunity presented itself that required a "temporary" relocation without promise of return. It has been an adventure including working on a timber framed barn by hand, reading books I never would have considered, and doing architectural work for a manufacturing company building hospitals. A year and a half later and I have finally gotten enough of a whisper of peace to begin again this beautiful hobby that demands ... stability! The twist is it's not true stability. I have a year and a half left on this lease (which is in Birmingham Alabama, a wonderful city but far cry from my beloved Chicago) after which I will relocate again - but I think that's enough time to grow something beautiful. While in Alabama I have observed some remarkable ecosystems and natural + unnatural landscapes. All of which kept this hobby on my mind. Something about desiring always to be near such landscapes, curious about the inhabitants they nurture, and aspiring to create. I'll begin with a few choice photos of the above.






I was looking forward to the direction of my last two aquariums and decided to pick up a similar thread with the two I am bringing to you now. The larger 45P aquarium to be a simple layout with lots of green, more focused on Utricularia Graminifolia and Epiphytes, the smaller, 30CM aquarium to be arranged more as a dutch garden with all of the stem plants my heart desires. I realized one of my biggest failing points on the last run, as short as it was, was the fact that both aquariums sat in windows and got some amount of direct sunlight every day. That, combined with the hard water of the city of Chicago and I think I set myself up for a struggle. For these reasons I chose a secluded room in my apartment with no windows to set up as the aquarium workshop. It used to be an elevator shaft in the center of the building, meaning it should be warmer during the winter and cooler in summer. I also invested in a 5 stage RO/DI filtration system from BulkReefSupply and fill up a 30 gallon drum regularly from the tap in my guest water closet.
For this run, I am using all of the same hardware- Oase Filtosmart 100 Thermo set to 74 degrees F on both / UNS MINI Dual Stage CO2 regulator on both / CO2 Art in-line diffuser on both / ONF Flat Nano+ for the 30CM cube and a Twister 45 S-series for the 45P. With an out-of-the-box flow rate of 160GPH, crazy bright lights and a solid CO2 system, I feel confident I have no hardware to blame for any of my impending shortcomings.
Because of my fascination with UG and deep desire to "get it right," I decided on a long dark start. This gave me time to get my hands on an RO system and think about my plant selections as well. I began this back in November, so it was a solid 2 months for both tanks. I started with dechlorinated tap and some Seachem prime which was running low. Whilst researching on bacterial supplements I came across this thread which gave me some strong reason to doubt the trusty system I have used so many times in the past. Further reading through this forum and I found this piece from another thread:





A few weeks later I acquired a lovely SW380T trinocular microscope from Swift and began exploring samples from the bottom of the aquarium. The desert I saw got me thinking about just how much life I would need crawling through the soil for the UG so I decided to visit a local pond to collect some detritus - old leaves etc, to add to the mix. This time I just dumped it all in, including some crushed up Catapa leaves I've had laying around.

I had introduced the RO/DI water, remineralized with APT Sky, to the system at this point and let the thing run for another several weeks. Finally, I did a water test, removed all of the detritus, and put in a large plant order. I started using a Hagen test kit from Nutrafin after inconclusive results from my old API test kits. The results of this 2 month dark start were encouraging.


Planting the 30CM Cube:

I hope to be setting myself up for success here. At this rate, it is simply up to my patience, attention to nutrient levels, and ability to balance lighting and CO2. As far as lighting is concerned, here is my lighting schedule on the ONF Flat Nano+
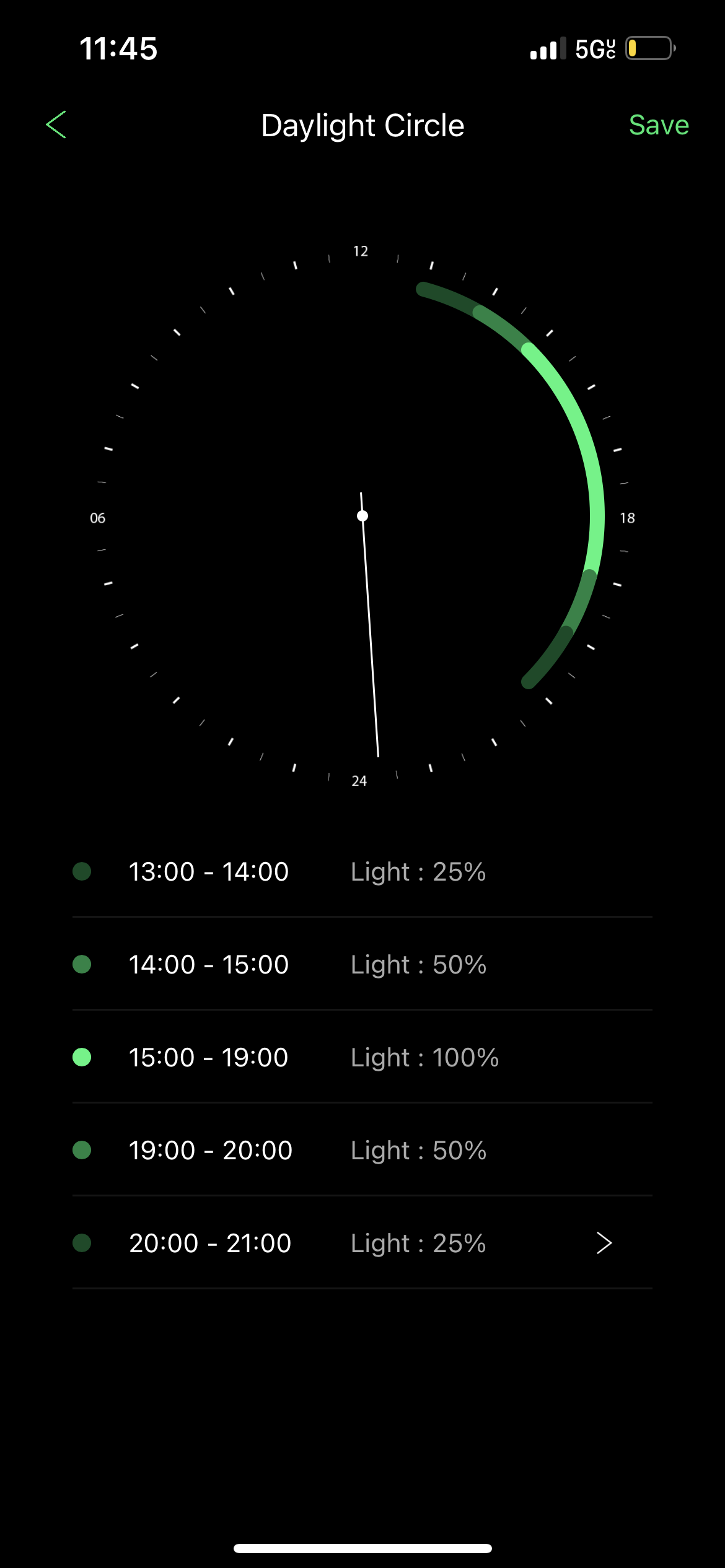
How do you feel about the above cycle for a fresh, heavily planted aquarium running CO2? For the Twister, I'm running it with a Way2Top dimmer system with 8 programable modes, (2 of which are set to zero to encapsulate the "off time", since the thing ramps continuously between modes until it hits the target brightness). I run it for 10 hours from 12-10PM, ramping from 0-25 for the first hour, 25-50 the second hour, and 50-100 the third hour, before sitting at 100 for 4 hours and ramping from 100-50 over the next hour, from 50-25 the hour after, and finally 25-0 for the last hour of the cycle. Does this seem like too much light for the new setup? I am considering dropping the first and last hour ramps down to 5%. Do you prefer a short period of intense light or an extended period of gradual and less intense light?
If you've made it this far, thank you for your time! Cheers,
Daniel
I was looking forward to the direction of my last two aquariums and decided to pick up a similar thread with the two I am bringing to you now. The larger 45P aquarium to be a simple layout with lots of green, more focused on Utricularia Graminifolia and Epiphytes, the smaller, 30CM aquarium to be arranged more as a dutch garden with all of the stem plants my heart desires. I realized one of my biggest failing points on the last run, as short as it was, was the fact that both aquariums sat in windows and got some amount of direct sunlight every day. That, combined with the hard water of the city of Chicago and I think I set myself up for a struggle. For these reasons I chose a secluded room in my apartment with no windows to set up as the aquarium workshop. It used to be an elevator shaft in the center of the building, meaning it should be warmer during the winter and cooler in summer. I also invested in a 5 stage RO/DI filtration system from BulkReefSupply and fill up a 30 gallon drum regularly from the tap in my guest water closet.
For this run, I am using all of the same hardware- Oase Filtosmart 100 Thermo set to 74 degrees F on both / UNS MINI Dual Stage CO2 regulator on both / CO2 Art in-line diffuser on both / ONF Flat Nano+ for the 30CM cube and a Twister 45 S-series for the 45P. With an out-of-the-box flow rate of 160GPH, crazy bright lights and a solid CO2 system, I feel confident I have no hardware to blame for any of my impending shortcomings.
Because of my fascination with UG and deep desire to "get it right," I decided on a long dark start. This gave me time to get my hands on an RO system and think about my plant selections as well. I began this back in November, so it was a solid 2 months for both tanks. I started with dechlorinated tap and some Seachem prime which was running low. Whilst researching on bacterial supplements I came across this thread which gave me some strong reason to doubt the trusty system I have used so many times in the past. Further reading through this forum and I found this piece from another thread:
I decided to scrap the bottled stuff altogether and do precisely that. I went out to a lake I enjoy, pulled out some of the plants growing along its perimeter, cut the roots and mixed with dechlorinated water in a bucket. This concoction was then poured equally into both aquariums with the aid of a strainer. This was perhaps 1 month into the dark start.I agree with Ian. You can get billions of bacteria for free by simply digging up the largest weeds you find in your garden and scraping the soil from the roots. Mix it into the substrate as well as mixing it into the filter media. Job done.
Cheers,
A few weeks later I acquired a lovely SW380T trinocular microscope from Swift and began exploring samples from the bottom of the aquarium. The desert I saw got me thinking about just how much life I would need crawling through the soil for the UG so I decided to visit a local pond to collect some detritus - old leaves etc, to add to the mix. This time I just dumped it all in, including some crushed up Catapa leaves I've had laying around.
I had introduced the RO/DI water, remineralized with APT Sky, to the system at this point and let the thing run for another several weeks. Finally, I did a water test, removed all of the detritus, and put in a large plant order. I started using a Hagen test kit from Nutrafin after inconclusive results from my old API test kits. The results of this 2 month dark start were encouraging.
- 6.6 PH
- 9 dGH
- <0.5dKH
- 0.00 ppm Iron (free or chelated)
- 0.00 ppm Ammonia
- 0.1 ppm Nitrite
- >10ppm Nitrate (just slightly more, for scientific accuracy ...)
- 0.25ppm Phosphate
- ~ 160ppm Calcium, which corresponds closely with the 9dGH measured earlier after converting ( divide by 17.8575...)
- 4 in vitro cups of Utricularia Graminifolia. I broke these into very small portions and planted them quite deep in the front 2/3rds of the aquarium.
- An assortment of mini Bucephalandra variations glued to the stone, including some brownie ghost, phantoms, mini reds, and a marbled variety.
- A few of these petite albino Anubias laughably called "White platinum petite". I am fascinated by them but don't have high hopes for their success.
- Several loose portions of Riccardia Chamedryfolia glued to the stone (with some other mosses mixed in ...).
- 2 bare root bundles of Lilaeopsis Brasiliensis (allegedly, who's to say it's not another variation of micro sword until it fills in ... I am not an expert on these) planted along the back of the aquarium.
Planting the 30CM Cube:
- 2 In vitro "bags" of Micranthemum Monte Carlo in the foreground. Much less than I wanted but should fill in just fine.
- 1 in vitro "bag" of Hygrophila SP Chai to mix into the foreground. I was getting some amount of growth before the move last time, they just had to compete with some larger neighboring plants, so I figured I might have better luck in a mixed foreground carpet?:
- 1 in vitro "bag" of Alternanthera Reineckii Mini on either side of a green bush
- 1 in vitro "bag" of Staurogyne Repens in the center
- 2 loose-cut bunches of emerged growth Rotala Florida in center behind the S. Repens
- 2 in vitro "bags" of Rotala Indica (bonsai) flanking the Florida on either side
- 1 in vitro cup of Rotala Macranda Mini Butterfly planted in a line behind the Florida and Indica
- 1 loose-cut bunch of emerged growth Ludwigia Arcuata just behind the Mini Butterfly, flanking on either side
- 1 loose-cut bunch of emerged growth Ludwiga Super Red just behind the Arcuata
- 2 loose-cut bunches of emerged growth Rotala Rotundifolia mixed close to the back
- 2 loose-cut bunches of emerged growth Rotala HRA mixed close to the back
- 2 loose-cut bunches of emerged growth Rotala Rotundifolia Green lining the back
I hope to be setting myself up for success here. At this rate, it is simply up to my patience, attention to nutrient levels, and ability to balance lighting and CO2. As far as lighting is concerned, here is my lighting schedule on the ONF Flat Nano+
How do you feel about the above cycle for a fresh, heavily planted aquarium running CO2? For the Twister, I'm running it with a Way2Top dimmer system with 8 programable modes, (2 of which are set to zero to encapsulate the "off time", since the thing ramps continuously between modes until it hits the target brightness). I run it for 10 hours from 12-10PM, ramping from 0-25 for the first hour, 25-50 the second hour, and 50-100 the third hour, before sitting at 100 for 4 hours and ramping from 100-50 over the next hour, from 50-25 the hour after, and finally 25-0 for the last hour of the cycle. Does this seem like too much light for the new setup? I am considering dropping the first and last hour ramps down to 5%. Do you prefer a short period of intense light or an extended period of gradual and less intense light?
If you've made it this far, thank you for your time! Cheers,
Daniel
Last edited:




