GraemeVW
Member
I have been using this as dechlorinator but ive not really been that accurate. Not had any problems, but thought maybe I should work out the dosage better.
Does anyone know how?
I found this link...
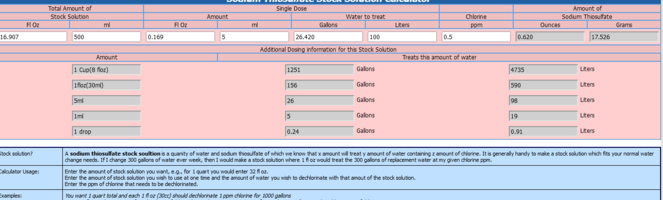
It suggests 4oz (100g) dissolved in almost 2 litres of water (I'd use deionised) would give me a solution that treats 1 us gallon with 1 drop of water.
The link suggests there are 100 drops in a teaspoon and a teaspoon treats 100 gallons.
I tend to treat 25 litres at a time, which I think works out to 7.5 drops.
Previously I'd just tipped a bit in, but I've been overdosing. Not caused me any problems though.
7.5 drops seems a tiny amount to add to 25 litres. I'm sure I read somewhere to double the dose if there is chloramine.
Anyone else use sodium thiosulfate?
Does anyone know how?
I found this link...
It suggests 4oz (100g) dissolved in almost 2 litres of water (I'd use deionised) would give me a solution that treats 1 us gallon with 1 drop of water.
The link suggests there are 100 drops in a teaspoon and a teaspoon treats 100 gallons.
I tend to treat 25 litres at a time, which I think works out to 7.5 drops.
Previously I'd just tipped a bit in, but I've been overdosing. Not caused me any problems though.
7.5 drops seems a tiny amount to add to 25 litres. I'm sure I read somewhere to double the dose if there is chloramine.
Anyone else use sodium thiosulfate?
Last edited: