Hi all,
I’m currently scratching my head trying to work this out. My average at best chemistry knowledge is being tested.
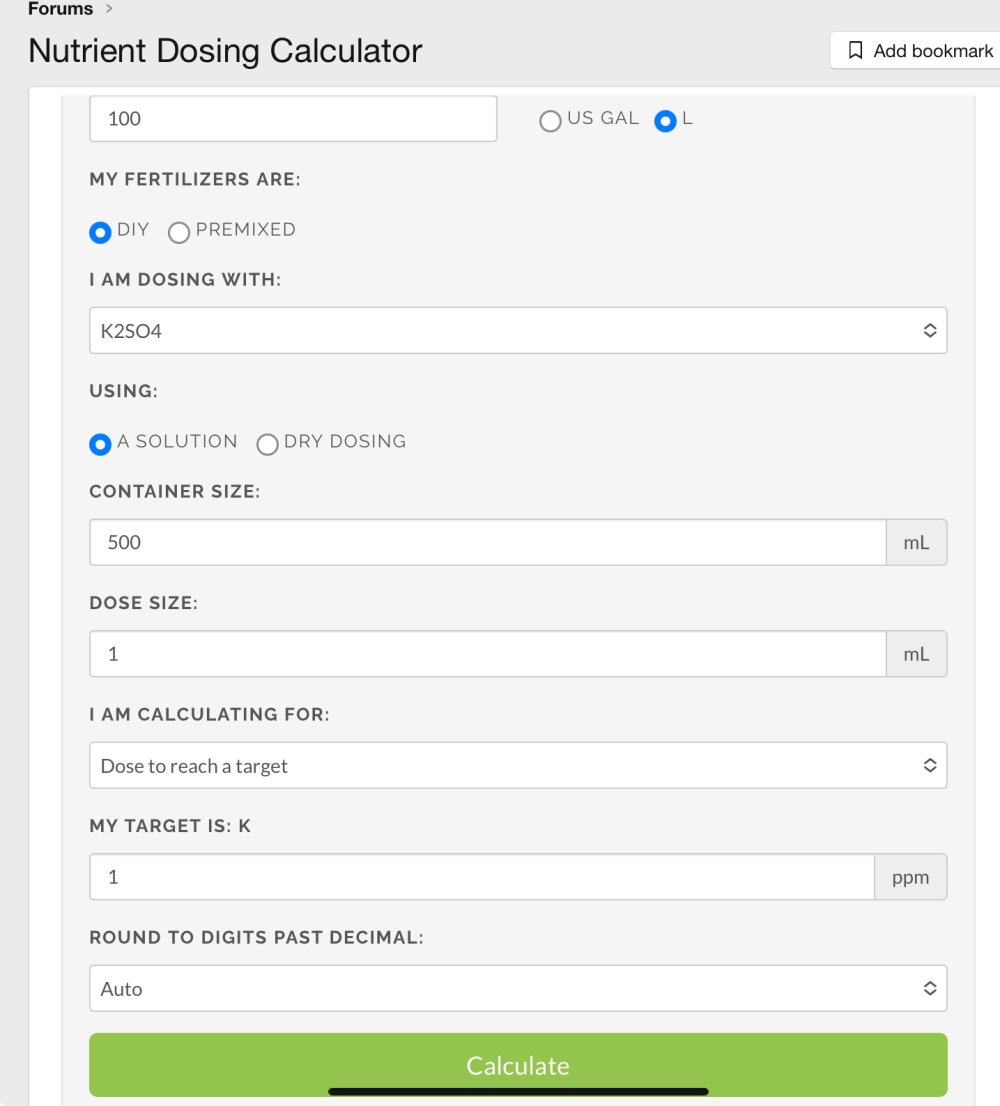
I’m looking to make a liquid solution from dry Potassium Sulphate to cure a potassium deficiency in my aquarium.
I’d like to pre-dissolve the dry powder in a water solution so that I can apply it to the aquarium via a dosing pump.
I’m looking to use something like this:
Potassium Sulphate
Now my question is how do I calculate how much dry potassium sulphate powder to add to 500ml of water to yeild a concentration of 1ppm of K per 100L of water after adding 1ml of the solution?
I’m not even sure that would be a workable concentration, but hopefully you get the idea. Unfortunately I’ve spent many years as an electrical engineer and chemistry is certainly not my strong point!
I’m currently scratching my head trying to work this out. My average at best chemistry knowledge is being tested.
I’m looking to make a liquid solution from dry Potassium Sulphate to cure a potassium deficiency in my aquarium.
I’d like to pre-dissolve the dry powder in a water solution so that I can apply it to the aquarium via a dosing pump.
I’m looking to use something like this:
Potassium Sulphate
Now my question is how do I calculate how much dry potassium sulphate powder to add to 500ml of water to yeild a concentration of 1ppm of K per 100L of water after adding 1ml of the solution?
I’m not even sure that would be a workable concentration, but hopefully you get the idea. Unfortunately I’ve spent many years as an electrical engineer and chemistry is certainly not my strong point!