-
You are viewing the forum as a Guest, please login (you can use your Facebook, Twitter, Google or Microsoft account to login) or register using this link: Log in or Sign Up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
How much carbon in natural waterways?
- Thread starter HiNtZ
- Start date
Keith GH
Member
Ask your water supplier that one and the answer would be official.
Keith

Keith
zozo
Member
In a nutshell..  It's originating source, such as sediment deposites it runs through.. In principle no matter if the source is a melting glacier or from rain fall, initialy the atmosphere is the originating source of all water on the planet.. Thus initialy it starts with water that has very little of everything in it only what it collects from the atmosphere during it's way down again. And once on the surface again mean while its way down to the lowest point it washes out everthing it runs through and over. It collects and accumulates numerous elements such as carbon sources from these sediments and is constantly changing in parameters along the way.
It's originating source, such as sediment deposites it runs through.. In principle no matter if the source is a melting glacier or from rain fall, initialy the atmosphere is the originating source of all water on the planet.. Thus initialy it starts with water that has very little of everything in it only what it collects from the atmosphere during it's way down again. And once on the surface again mean while its way down to the lowest point it washes out everthing it runs through and over. It collects and accumulates numerous elements such as carbon sources from these sediments and is constantly changing in parameters along the way.
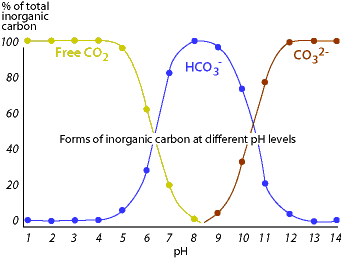
No idea what you are aiming towards with the question.. I assume you aim towards CO² than it also relates to acidity and carbonates. The so called gH and KH, calcium carbonates and Natrium bicarbonates etc. Than for example if water runs through peat sediments it gets more acidic from other elements it collects and gets a higher pH. In higher pH the carbonate molecules split and release CO². In lower pH it rather stays a carbonate molecule. But for this more in depth explaination, you have to wait for more expertised members finding your question willing to go into the subject.
No idea what you are aiming towards with the question.. I assume you aim towards CO² than it also relates to acidity and carbonates. The so called gH and KH, calcium carbonates and Natrium bicarbonates etc. Than for example if water runs through peat sediments it gets more acidic from other elements it collects and gets a higher pH. In higher pH the carbonate molecules split and release CO². In lower pH it rather stays a carbonate molecule. But for this more in depth explaination, you have to wait for more expertised members finding your question willing to go into the subject.
There's a good bit about this in Diana Walstad's book Ecology of the Planted Aquarium. I think the gist of it is that carbon is scarce in freshwater and levels fluctuate rapidly. CO2 levels typically range from 0 to over 14 mg/l. Most CO2 is generated by decomposition of POC (particulate organic carbon) and DOC (dissolved organic carbon).
Like Marcel says pH also affects CO2 concentration, it determines the relative proportions of CO2, bicarbonates and carbonates. Basically, the lower the pH the higher the percentage of DIC (dissolved inorganic carbon) that exists as CO2.
Like Marcel says pH also affects CO2 concentration, it determines the relative proportions of CO2, bicarbonates and carbonates. Basically, the lower the pH the higher the percentage of DIC (dissolved inorganic carbon) that exists as CO2.
zozo
Member
A nice safe home experiment with Acid and Carbonates in relation to CO² is overdoing it compaired by natural levels with making your home brew sodapop limonade. The way it was made in the first half of the 20th century. Than take 1 litre of tap water in a pet bottle (Sweetened with syrup or not) and gently throw in 12 grams of Natrium Bicarbonate (baking soda) followed by 12 gram of Citric acid powder. (Or other way around) and it starts reacting immediately producing CO² bubbles, quickly screw on the cap put it in the fridge and waiit a day for a delicious bottle of sprankling sweet limonade. 
Dennis Wong has compiled co2 in natural waters. The sample size is small which shows that 10 mg/l is most common, <5 and >30 are less common.
https://www.advancedplantedtank.com/choosing-co2-why.html
https://www.advancedplantedtank.com/choosing-co2-why.html
Edvet
Member
Don't forget in nature it gets replaced 24/7 , so it doesn't run out, and if we see healthy plantgrowth in nature it has been selected through natural selection to flourish where they grow (otherwise we see no plants).
The beautifull anf full grown places like Bonito are being fed through subteranean aquafers and springs,



The beautifull anf full grown places like Bonito are being fed through subteranean aquafers and springs,
rebel
Member
- Joined
- 4 Aug 2015
- Messages
- 2,269
Clear water habitats are very rare anywhere. I believe 1% of all freshwater or even less.Those Pics are too beautiful to be real. Clear water habitats are rare in the Amazon. 95% of the water there are black or white water with low visibility, and if there are any plants, they are dominated by one to very few species.
I am also curious how low CO2(presumbly - has anyone measured the actual CO2 amounts) and high light doesn't seem to cause algae. There must be other unknown variables in this equation.
Edvet
Member
1) most habitats have none or little plants
2) plants grow where they can adapt
3) lightlevels are mostly low ( filtered and blocked through the canopy) ( we see pics of mid day situations where sun is from above)
4) lots of floaters, much much more ( floating meadows) compared to submersed plants
5) often when we see submerged plants its in flooded areas ( waters can rise 10-20 meter in places)
6) most of the times submerged plants are a few leaves struggling not to be covered in algae/debris (look throuh the hours of video from Mikolji, and he is even driving for hours and hours to find filmable waters)
7) the continuous refreshment of all nourishment and fertilisation is not to be compared to a closed tank.
https://www.frontiersin.org/articles/10.3389/fmars.2017.00076/full
https://eos.org/research-spotlights/why-is-there-so-much-carbon-dioxide-in-rivers
https://phys.org/news/2015-08-significant-streams-rivers.html
2) plants grow where they can adapt
3) lightlevels are mostly low ( filtered and blocked through the canopy) ( we see pics of mid day situations where sun is from above)
4) lots of floaters, much much more ( floating meadows) compared to submersed plants
5) often when we see submerged plants its in flooded areas ( waters can rise 10-20 meter in places)
6) most of the times submerged plants are a few leaves struggling not to be covered in algae/debris (look throuh the hours of video from Mikolji, and he is even driving for hours and hours to find filmable waters)
7) the continuous refreshment of all nourishment and fertilisation is not to be compared to a closed tank.
https://www.frontiersin.org/articles/10.3389/fmars.2017.00076/full
https://eos.org/research-spotlights/why-is-there-so-much-carbon-dioxide-in-rivers
https://phys.org/news/2015-08-significant-streams-rivers.html
zozo
Member
Clear water habitats are very rare anywhere. I believe 1% of all freshwater or even less.
I am also curious how low CO2(presumbly - has anyone measured the actual CO2 amounts) and high light doesn't seem to cause algae. There must be other unknown variables in this equation.
Never realy measured it, don't know if it can be measured that accurately.. But regarding the kh ph table, my outdoor Mission Bathtub setups are pH 8,5 in highlight period and kH 10. That's less than 1.3ppm CO² according the table.. Even if the table aint accurate it can't be so much more or less, but i have no algae issues other than Clado which more is a plant than an algae actualy. Even the glass of the little aqaurium stays remarkably clean, only cleaned it twice this year with very little algae on it.. The submersed potamogeton and Lileaopsis b. and hairgrass growing realy good and stay clean.
Hi all,
The reason that temperature is important is that it regulates the solubility of gases and it is the CO2 concentration in the atmosphere that governs everything else.
If you have a source of added CO2 you can naturally get huge CO2 concentrations, I've just returned from a month in Rotorua, where volcanic CO2 release leads to water with very low pH and large amounts of dissolved CO2. This process is documented in the <"Lake Nyos disaster">.
For Dissolved Organic Carbon (DOC), the natural level ranges from trace amounts to about 10% of the water volume. We've been doing some work in deep disused limestone quarry voids, and in the more recently abandoned of these you have very clear water with visibility of ~10 metres and very little in the way of DOC or nutrients (the water has plenty of HCO3- and Ca++ ions, but nothing else).
After that the nature of the DOC matters, you are back to old friend <"Biochemical Oxygen Demand (BOD)">. You could have very low nutrient tannin rich water where you have very low BOD, or you could have organic rich very polluted water with an immense BOD (This is covered in some detail in the <"So what is organic..."> thread).
cheers Darrel
You have to specify which type of carbon you are talking about. If you just take "Dissolved (or Total) Inorganic Carbon" "TIC/DIC" in nearly all circumstances you have the same amount, dependent upon temperature, and assuming that you have 400ppm CO2 in the atmosphere. All that changes <"is the form it is in">.As per title - let's assume the highest concentration possible....
The reason that temperature is important is that it regulates the solubility of gases and it is the CO2 concentration in the atmosphere that governs everything else.
If you have a source of added CO2 you can naturally get huge CO2 concentrations, I've just returned from a month in Rotorua, where volcanic CO2 release leads to water with very low pH and large amounts of dissolved CO2. This process is documented in the <"Lake Nyos disaster">.
For Dissolved Organic Carbon (DOC), the natural level ranges from trace amounts to about 10% of the water volume. We've been doing some work in deep disused limestone quarry voids, and in the more recently abandoned of these you have very clear water with visibility of ~10 metres and very little in the way of DOC or nutrients (the water has plenty of HCO3- and Ca++ ions, but nothing else).
After that the nature of the DOC matters, you are back to old friend <"Biochemical Oxygen Demand (BOD)">. You could have very low nutrient tannin rich water where you have very low BOD, or you could have organic rich very polluted water with an immense BOD (This is covered in some detail in the <"So what is organic..."> thread).
cheers Darrel
Hi all,
There is probably naturally CO2 enriched clear water in @zanguli-ya-zamba's <"DR Congo expedition..."> thread.
cheers Darrel
There is probably naturally CO2 enriched clear water in @zanguli-ya-zamba's <"DR Congo expedition..."> thread.
cheers Darrel
In my planted shrimp bowl that receives 4 hr direct sunlight, estimated co2 at peak light period is 0.6 ppm at kH6 and pH8.5. There is no algae of any sort, so Barr’s theory that high co2 will solve 90% of algae problem is not substantiated. I would propose that high intensity light equivalent to sunlight level will solve algae problem.Clear water habitats are very rare anywhere. I believe 1% of all freshwater or even less.
I am also curious how low CO2(presumbly - has anyone measured the actual CO2 amounts) and high light doesn't seem to cause algae. There must be other unknown variables in this equation.
Unlike coral reef and kelp forest scenes, lush planted clear water habitats are extremely rare in freshwater. Clear water alkaline lakes in African Rift Valley have abundant algae, but not much plant growth,if any. Most freshwater scents have murky or tannic water with poor visibility, detritus covered wood and rock, and worse, human trash. Lush planted tanks are artificial sensations rarely duplicated in nature, and vice versa with reef tanks.
Edvet
Member
We (majority of this forum) tend to think it is; the majority of the problems we see are: lots of algea, lots of light, insufficient CO2 levels and distribution. improving he latter will solve the first.There is ample evidence that is a functional solution. Barr's theory is a good one and i haven't seen any contradictory evidence against it. If you have any evidence ( scientific or through well documented journals) please share.high co2 will solve 90% of algae problem is not substantiated.
Hi all,
Another factor is that the division between the plants you want and the plants you don't ("algae") is an entirely artificial one.
If you have light, water and even a trace of nutrients plants will grow. Green Algae belong to the same clade (<"Viridiplantae">) as all the mosses, fern and higher plants we might have planted. There isn't one state that favours "algae", there are algae that grow under a huge range of different growing conditions.
Some plants (both algae and higher plants) will have high potential growth rates, they basically want/need everything (light and nutrients) <"turned up to 11">, others will grow in sub-optimal conditions.
If you <"don't mind slow growth"> you can use mosses, ferns, Cryptocoryne spp., Anubias etc. in tanks with minimal nutrient addition.
cheers Darrel
They are an artificial construct, but I think partially this is due to <"anthropogenic effects">, and that if you could go back in time 1,000 years you would find a lot more fresh water bodies with clear water.Unlike coral reef and kelp forest scenes, lush planted clear water habitats are extremely rare in freshwater. Clear water alkaline lakes in African Rift Valley have abundant algae, but not much plant growth,if any. Most freshwater scents have murky or tannic water with poor visibility, detritus covered wood and rock, and worse, human trash. Lush planted tanks are artificial sensations rarely duplicated in nature
Another factor is that the division between the plants you want and the plants you don't ("algae") is an entirely artificial one.
If you have light, water and even a trace of nutrients plants will grow. Green Algae belong to the same clade (<"Viridiplantae">) as all the mosses, fern and higher plants we might have planted. There isn't one state that favours "algae", there are algae that grow under a huge range of different growing conditions.
Some plants (both algae and higher plants) will have high potential growth rates, they basically want/need everything (light and nutrients) <"turned up to 11">, others will grow in sub-optimal conditions.
If you <"don't mind slow growth"> you can use mosses, ferns, Cryptocoryne spp., Anubias etc. in tanks with minimal nutrient addition.
cheers Darrel
I am not contradicting Barr. but want to point out that what we observe in nature is very different from in the glass box, and our understanding of what trigger algae is incomplete. According to Barr, low CO2 and intense light is a recipe for algae. It didn't happen to me and others who have tanks exposed to sunlight. Plants seem to be able to utilize low CO2 very efficiently given sunlight which is many times more intense that the highest artificial light. The 30 ppm CO2 target in high tech tank is rare in nature. There is also under measurement of CO2 by pH probe due to abundant free CO2(mist) generated by diffuser, making total CO2 availability to submerged growth greater than atmospheric CO2 to emerged growth.We (majority of this forum) tend to think it is; the majority of the problems we see are: lots of algea, lots of light, insufficient CO2 levels and distribution. improving he latter will solve the first.There is ample evidence that is a functional solution. Barr's theory is a good one and i haven't seen any contradictory evidence against it. If you have any evidence ( scientific or through well documented journals) please share.
Last edited:
Hi all,
cheers Darrel
I should have linked in the Lenntech article <"General Effects of Eutrophication">, it shows the changes in algal assemblage as nutrient levels rise.There isn't one state that favours "algae", there are algae that grow under a huge range of different growing conditions.
cheers Darrel
Hi all,
This photo is of a <"bucket full of Hornwort with a resident frog">, it is in full sun and if it didn't have a frog in it it would have a small amount of Hornwort and a lot of green filamentous algae.
The frog will be supplying some CO2, but I assume the effect is mainly a nutrient one.

cheers Darrel
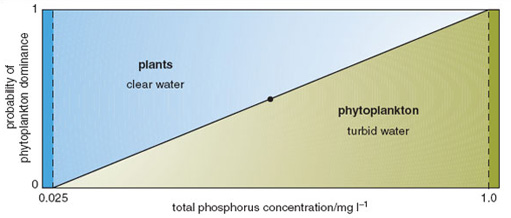
This is certainly true in some situations, this also occurs in the natural environment where water bodies may <"remain in a macrophyte dominated state"> long after the level of nutrients would have usually led to a phytoplankton dominated state.It didn't happen to me and others who have tanks exposed to sunlight. Plants seem to be able to utilize low CO2 very efficiently given sunlight which is many times more intense that the highest artificial light.
Figure 3.5: Probability plot of two stable states in shallow freshwater ecosystems. Over a broad range of phosphorus concentrations in the eutrophic-hypertrophic range, either state may potentially occur. However, once established, that state promotes processes that result in it becoming stabilized, and switches between the two states are only rarely observed.
This photo is of a <"bucket full of Hornwort with a resident frog">, it is in full sun and if it didn't have a frog in it it would have a small amount of Hornwort and a lot of green filamentous algae.
The frog will be supplying some CO2, but I assume the effect is mainly a nutrient one.
cheers Darrel


