Hi, folks! What's the correct equilibrium concentration of CO2 in water with atmosphere? I read in Diana Wastad book's (Ecology of planted aquarium) that it's about 0,5 ppm. But most people say it's 3 ppm. I'm quite confused. So, it's 0,5 or 3? Thank you.
-
You are viewing the forum as a Guest, please login (you can use your Facebook, Twitter, Google or Microsoft account to login) or register using this link: Log in or Sign Up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
CO2 gaseous equilibrium with atmosphere
- Thread starter Victor
- Start date
Zeus.
Fertz Meister
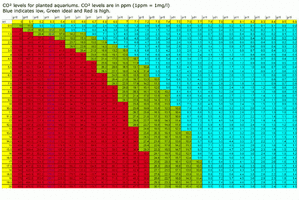
Graph doesnt have 22 degrees but 0.5-1.0ppm CO2 would be my estimate, but with poor flow, high plant mass and lights on it will soon drop
Zeus.
Fertz Meister
Plus I just notice your from Brazil so maintaining 22 degrees may be difficult so with increasing water temps less [CO2] again
Yes, it makes sense. My tank water is 22 degrees. I have a very strong surface agitation to allow a extremely high CO2 injection rate. The ph of my tank rises to about 8 some hours after CO2 turns off (I'm using test kit to measure ph because my ph probe is fauty) . My kh is 3. During CO2 saturation peak at photoperiod the ph drops to 6.6. So the 1 ph drop rule to get 30 ppm of CO2 isn't correct? It's actualy a lot more, not? So let's suppose the CO2 equilibrium point is really 0.5 ppm, I need to move from ph 8,3 to ph 6,4 to get about 36 ppm of CO2. It's a 1,9 ph drop! Is that correct?
Attachments
I use a chiller to keep this cooler temperaturePlus I just notice your from Brazil so maintaining 22 degrees may be difficult so with increasing water temps less [CO2] again
Zeus.
Fertz Meister
I would go off the DC colour change, which if you have a 1.9pH drop the DC will be nearly clear IMO
I'm using a DC. It changes its color from deep blue to green in about 2- 4 hours and keep green until the CO2 turns off. I'm planning to drop the carbonate hardness (KH) to just 1 degree because my shrimps prefer very low kh. Is there some risk to CO2 become toxic at low ph coupled with low kh? I'm asking because I read this in Diana Walstad book:I would go off the DC colour change, which if you have a 1.9pH drop the DC will be nearly clear IMO
"Question: In the store we have a 40 gal plant tank. pH is about 7.2. Only R.O. (reverse osmosis) water is used. I have recently added CO2 injection to this system, and it seems to have made a positive difference for the plants. However, the visual tester usually indicates there is too much CO2 in the water. I have switched to mouth-breathing fish (gouramis and bettas), because the Kribensis and Congo tetras were gasping at the surface. Under these circumstances, is it bad to slightly overdose on CO2? Answer: Your fish are in grave and imminent danger. Adding CO2 to R.O. water can easily kill them. (R.O. water contains almost no salts, so the water would not have enough bicarbonate salt to buffer the added CO2 .) If you use CO2 injection, you simply must maintain a certain alkalinity in the water. The addition of hard tapwater or baking soda are ways to increase alkalinity. One of these additions, which must be done on a periodic basis, should bring the alkalinity up to normal test levels. Your tank should have a carbonate hardness (KH) above 3 or 4."
So should I be concerned about low kh in CO2 injected tanks?
Alex121
Seedling
I would love to know the answer to you original question as well @Victor, researching on the internet and at @Zeus' graph both suggest around 0.5ppm. My best guess is the 1PH drop has become recommended based on experience since around 0.5ppm is a theoretical equilibrium between air and pure water. Planted tanks are far from pure water and of course the plants are releasing CO2 overnight so the co2 levels in the morning are unlikely to have completely degassed. I have taken a water sample and left it for 24 hours and the PH is noticeably higher than my tank PH in the morning. With regards to your low KH question I live in birmingham which has extremely soft water sometimes registering 0 using my API KH test kit, I have never had any problems but do watch things very closely when dialling in CO2, I have noticed Seriyu stone is very good at giving some KH, I have some in my big tank and the KH usually tests somewhere between 4-6.
Hi all,
We aren't entirely sure where the "3ppm value" came from, but there are threads about how it relates to the <"dKH/CO2/pH chart"> and the <"original 3 ppm reference">.
cheers Darrel
It is 0.6 ppm if you work from <"Henry's Law and the 415 ppm of CO2 in the atmosphere">.What's the correct equilibrium concentration of CO2 in water with atmosphere? I read in Diana Wastad book's (Ecology of planted aquarium) that it's about 0,5 ppm. But most people say it's 3 ppm. I'm quite confused. So, it's 0,5 or 3?
We aren't entirely sure where the "3ppm value" came from, but there are threads about how it relates to the <"dKH/CO2/pH chart"> and the <"original 3 ppm reference">.
cheers Darrel
Last edited:
AverageWhiteBloke
Member
I don't think so co2 is a very weak acid, your ph will be lower in softer water but still have the same amount of co2 as a higher ph tank with higher KH water. I don't think anyone would use pure RO water in a tank.So should I be concerned about low kh in CO2 injected tanks?
Hi @VictorI read in Diana Wastad book's (Ecology of planted aquarium) that it's about 0,5 ppm.
As you have a copy of Diana Walstad's book, you may also find it interesting to read "Decomposition in the Sediment as a CO2 Source", which is on page 60 of the Third Edition. The point she makes is that decomposition of organic matter in the substrate releases both carbon dioxide (CO2) and methane (CH4). So, I guess if you are using a soil-based substrate, it will release these gases. If so, then CO2 concentration in the water column could be in excess of that purely due to atmospheric CO2. Indeed, even if not using a soil-based substrate, other organic matter (e.g. detritus) may slightly increase dissolved CO2. Surface agitation of the water and absorption of CO2 by plants will obviously have some bearing on how much CO2 is retained.
JPC
I have soil in my tank. But it's a very old soil, more than 5 years. I think it doens't produce CO2 anymore. I have only 20-25 crystal red shrimp in a 270 L tank. So I think the water CO2 concentration is reaching the equilibrium point of 0.5 ppm in my tank. In this case I'll aim for a 1,8 ph drop by injecting CO2. I hope it's a good ideaHi @Victor
As you have a copy of Diana Walstad's book, you may also find it interesting to read "Decomposition in the Sediment as a CO2 Source", which is on page 60 of the Third Edition. The point she makes is that decomposition of organic matter in the substrate releases both carbon dioxide (CO2) and methane (CH4). So, I guess if you are using a soil-based substrate, it will release these gases. If so, then CO2 concentration in the water column could be in excess of that purely due to atmospheric CO2. Indeed, even if not using a soil-based substrate, other organic matter (e.g. detritus) may slightly increase dissolved CO2. Surface agitation of the water and absorption of CO2 by plants will obviously have some bearing on how much CO2 is retained.
JPC
Hi @VictorIn this case I'll aim for a 1,8 ph drop by injecting CO2. I hope it's a good idea
I'm not sure that's a good idea. If the starting CO2 concentration was 3 ppm (i.e. no injection) and if you were able to achieve a pH drop of 1.8, I reckon that would result in a CO2 concentration of 189 ppm!! A pH reduction of 1.0 with a starting CO2 concentration of 3 ppm will result in a CO2 concentration of 10X the starting point, i.e. 30 ppm. I have some reservations about adopting a 1.0 pH drop but that's accepted as 'the norm'. This is based on what we know about fish. I'm not sure about the tolerance level of shrimp to CO2. Anyway, if you're interested:
Why Advise A 1pH Drop?
Hi Folks, I don't understand why a 1pH drop is advised when setting up a profile for injected CO2. For example, if the water KH is 4dH and the starting pH is 7.3, the CO2 concentration will be approximately 6 ppm. But, after a 1pH drop to 6.3, the CO2 concentration will be a staggering 60 ppm...
www.ukaps.org
JPC
Hi Darrel,Hi all,
It is 0.6 ppm if you work from <"Henry's Law and the 415 ppm of CO2 in the atmosphere">.
We aren't entirely sure where the "3ppm value" came from, but there are threads about how it relates to the <"dKH/CO2/pH chart"> and the <"original 3 ppm reference">.
cheers Darrel
Is it possible that Henry's law work only for ideal solutions ?
Or it might not apply to CO2 because it reacts with water to form H2CO3 and derivatives, and it is also bound loosely to H2O with polar interactions. The calculated value using Henry's law is around 0.5 ppm, but that does not match the charts and CO2 calculators. Also might be that the CO2-measuring methods that were used to determine the solubility values, which give the 0.5 ppm concentration, and the ones used to determine the 3 ppm values were different, the latter also calculating with some bound (but otherwise available) forms of CO2.Hi Darrel,
Is it possible that Henry's law work only for ideal solutions ?
In the end, I don't think it matters if we calculate with 0.5 or 3 ppm values, it just refers to the CO2 concentration that is in equilibrium with air. We just need to take different constants in the equations if we use these values to calculate pH from CO2/KH values or CO2 from pH/KH. 10x change in dissolved CO2 will cause a 1 pH change regardless of the exact CO2 concentration.
Hi all,
Cheers Darrel
There is another thread somewhere that talks about CO2 disassociation. I'll see if I can find itHi Darrel,
Is it possible that Henry's law work only for ideal solutions ?
Cheers Darrel
Andy Pierce
Member
It does apply to CO2. The other forms of carbonate species, bicarbonate and carbonate, are determined in the equilibrated system by pH but the level of dissolved CO2 gas remains constant. Non-equilibrium factors can affect dissolved CO2 levels, including for example respiration by animals, photosynthesis by plants, decomposition of organic matter and of course, delibrately bubbling CO2 gas into the water column. Absent any active processes, dissolved CO2 is heading for 0.5 ppm.Or it might not apply to CO2 because it reacts with water to form H2CO3 and derivatives, and it is also bound loosely to H2O with polar interactions.
This is correct.10x change in dissolved CO2 will cause a 1 pH change regardless of the exact CO2 concentration.
Interesting, I always thought that this 3 ppm was the CO2 dissolved under equilibrium purely with air without any biological activity. It also matched approximately my pH/KH measurements, even in jars left in the air for a day or two, so I did accept these levels. It could be though that reaching equilibrium is a slower process, especially at lower CO2 levels...Absent any active processes, dissolved CO2 is heading for 0.5 ppm.
Hi all,
Cheers Darrel
We don't actually know where the 3 ppm CO2 figure comes from.Interesting, I always thought that this 3 ppm was the CO2 dissolved under equilibrium purely with air without any biological activity. It also matched approximately my pH/KH measurements, even in jars left in the air for a day or two, so I did accept these levels. It could be though that reaching equilibrium is a slower process, especially at lower CO2 levels...
Cheers Darrel