Gluteraldehyde is a highly reactive compound and it's effectiveness as a biocide means that it reacts with proteins and enzymes to disrupt their chemical structure. But the chemical behavior of glut is not really well understood. Also, there are other compounds which react with and which immobilize glut. In a tank there are lots of protein structures so this might be one reason it has a short half life. The part of the protein that glut is attracted to is the Amine functional group.
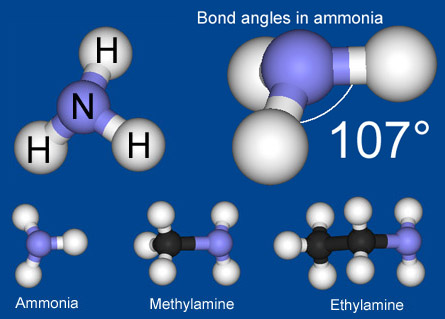
Amine is a derivative of ammonia. In the upper half of this image you can see Ammonia (NH3). If you pull one of the Hydrogen atoms away and replace it with some other element or compound then it becomes an Amine. Famous Amine structures are Amino Acids, which are the building blocks of proteins and enzymes.
There are other chemicals that react with glut depending on the pH and concentration. Dow Chemicals recommends that for glut spills, again, depending on concentration, the chemical can be neutralize with Sodium Bisulfite or Sodium Hydroxide. This is just a short list of way that can shorten the half life of this compound. There are many other chemical (organic and inorganic) paths. In a micro mix, normally the trace metals don't react with the glut, distilled or tap water is used (not tank water) and any organic baddies that develop are quickly rubbed out. So the chemical should have longevity in the bottle. Probably the same in an NPK mix, but again I don't really know because of the many ways that glut behaves in an aqueous solution.
Cheers,
Always a pleasure getting answers off you.

So i'm left with a feeling that for the trace mix people are thinking it will be fine with higher levels of Glut but the NPK mix is still up in the air especially when we add higher concentrations.
Ive read elsewhere people reporting their NPK solutions changed colour when the Glut was added (reddish tint) but they didnt report any negative dosing effects. But obviously something was happening.
As initially stated without testing we can't be sure.
On a side note i'm not sure if it's been mentioned enough in this thread how utterly horrible and harmful Glutaraldehyde is so here is a video i just found..... (it's not that interesting really).
Randomly while reading up I've just come across these articles.
http://www.safe.nite.go.jp/english/risk/pdf/03_summary/066sum.pdf
That one is a risk assessment in japan on glut with details of effects in water...
"(In air)
Reaction with OH radical:
Reaction rate constant is 8.40*10
-121
cm
3
/molecule-sec. (25degC, measured value)
The half-life is 8-20 hours, given OH radical concentration of 5*10
5
– 1*10
6
molecule/cm
3
.
Reaction with ozone:
The data is not available.
Reaction with nitrate radical:
The data is not available.
(In water)
Glutaraldehyde is expected to be hydrolyzed easily in basic water, and also has the possibility to
be oxidized by dissolved oxygen to produce glutaric acid.
Environmental fate
If released into water, glutaraldehyde is expected to be removed by biodegradation mainly under
aerobic conditions. Under anaerobic conditions, only primary degradation may occur."
The hazard assessment has a nice bit too showing concentrations needed for acute harm to algae, fish etc...
John Kiernan's web page - Formaldehyde and glutaraldehyde
A wider paper discussing the glut's reactions with aimino protien groups.
The Dow site seems to indicate nitrates and glut don't mix well and mention a colour change in an small note talking about corrosion inhibitors.
Dow Perf Materials & Basic Chem Answer Center
"What can you tell me about the compatibility of glutaraldehyde-based products with common corrosion inhibitors?
Typical anticorrosion agents such as azides, nitrates and nitrites can interact with glutaraldehyde (GA) and may result in effects such as color formation which could be intense at times. Chelants, such as Dow's VERSENETM products, are compatible with GA and are typically effective as corrosion inhibitors.
In addition, GA is known to be incompatible with:
- ammonia
- primary amines
- secondary amines at high concentrations
On the other hand, GA is generally quite compatible and very useful in combination with:
- secondary amines at low to moderate concentrations
- tertiary amines
- quaternary ammonium compounds
As always, the stability of formulations containing GA should be evaluated to insure that your performance criteria are met.
TMTrademark of The Dow Chemical Company ("Dow") or an affiliated company of Dow
So current thinking is still two dosing pumps needed

I'll keep reading or just make the experimental tank myself. Unfortunately i've only got the low intensity one to try it on, that is currently running a CO2 set up as an experiment.
I'm going to play with some plants and my head is hurting from all this high level chemistry reading.
Thanks again,
John


