Cornelius
Member
I sometimes read that the standard KH testst in our tanks can't really be reliable. That would be because our the standard test kits don't measure the KH but only alikinetics
It seems that the presence of other substances in our water can influence the KH. Such as phosphate, nitrate, silicate, ammonia, certain types of wood.
But to what extent are these substances decisive for a deviation from our test kits? I myself think that it is negligible, but what do you guys think about this?
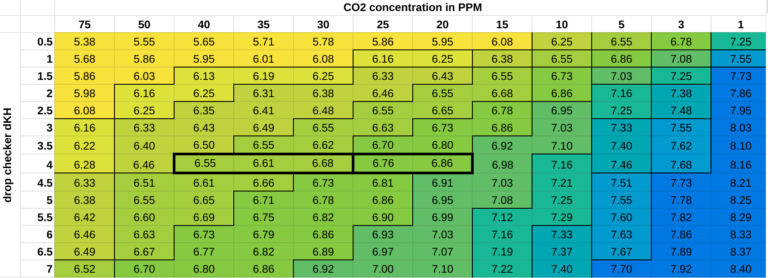
(I am asking this question in relation to adjusting the CO2 by a prope - pH-KH determination and the credibility to achieve a set amount of CO2)
cheers, Cor
It seems that the presence of other substances in our water can influence the KH. Such as phosphate, nitrate, silicate, ammonia, certain types of wood.
But to what extent are these substances decisive for a deviation from our test kits? I myself think that it is negligible, but what do you guys think about this?
(I am asking this question in relation to adjusting the CO2 by a prope - pH-KH determination and the credibility to achieve a set amount of CO2)
cheers, Cor