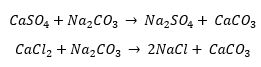Hi all,
Also, strange question, but is this the water that has been through an <"ion exchange water softener">? One that you fill up with salt (NaCl)?
cheers Darrel
The highish dKH will mean that iron (Fe++(+)) ions are mopped up to form insoluble hydroxide and carbonate, which chelator are you using?I have a low gH (2-3) and higher kH (~9) coming from my tap which I think may have been causing issues with iron uptake.
Also, strange question, but is this the water that has been through an <"ion exchange water softener">? One that you fill up with salt (NaCl)?
cheers Darrel