Barbara Turner
Member
I'm playing with a data logger and PH sensor and getting some strange results, I wondering if anyone has seen anything similar.
Each blue dot is based on a 100 readings that are then averaged. I take a new set of readings every 5 seconds.
The data below looks at a period of 5 hours.
The PH sensor was borrowed from a kedida CT-6020A
The water I'm measuring is RO water that's sat on my desk for 2 weeks in a glass tub.
I've checked the data logger against a calibrated fluke multi meter and it seems to be behaving itself.
I'm guessing at some point the sensor will level out.
I need to try and work out if it's the data logger or the sensor now..
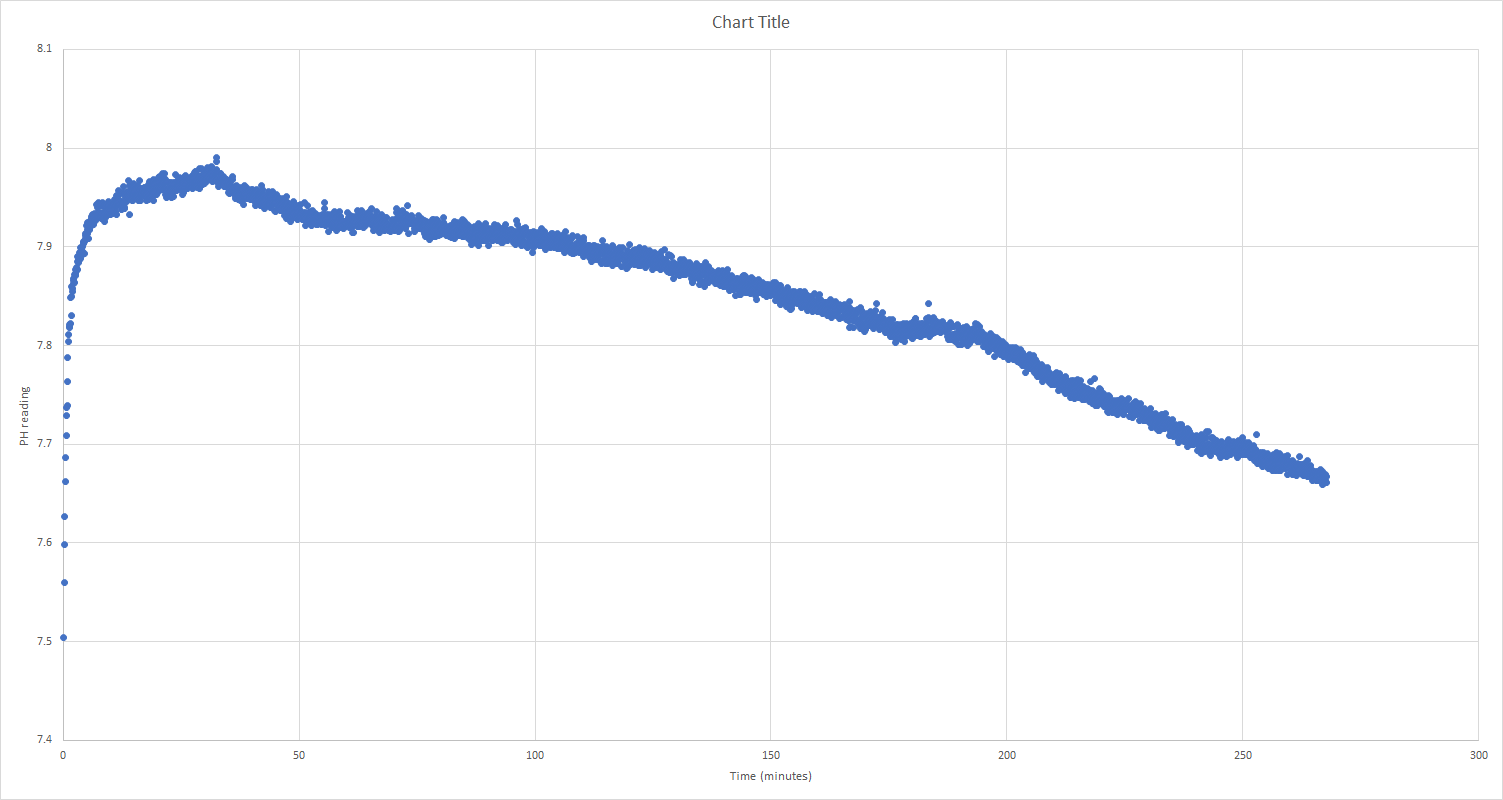
Each blue dot is based on a 100 readings that are then averaged. I take a new set of readings every 5 seconds.
The data below looks at a period of 5 hours.
The PH sensor was borrowed from a kedida CT-6020A
The water I'm measuring is RO water that's sat on my desk for 2 weeks in a glass tub.
I've checked the data logger against a calibrated fluke multi meter and it seems to be behaving itself.
I'm guessing at some point the sensor will level out.
I need to try and work out if it's the data logger or the sensor now..
Last edited:




